HOVON HO72
Archived
Main info
- Identifier:
- HOVON 72 AML
- Sponsor:
- HOVON
- Included patients:
-
2
- Active sites:
-
1(of 1)
- Title:
Dose escalation trial of bortezomib with standard remission-induction chemotherapy in patients with relapsed acute myelocytic leukemia (AML) or refractory anemia with excess of blasts (RAEB, RAEB-t) with IPSS score >= 1.5
Timeline
Scheduled
Actual
2005
28 Dec
Activated
2006
15 Nov
CloseoutInProgressLastPtOutActualStart
2016
15 Nov
Archived
Flow
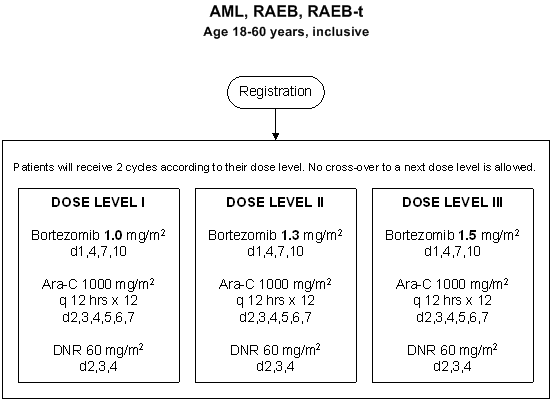
Details
- Phase:
- Prospective Phase I/II study
- Monitoring Type:
- Objectives:
- Evaluation of the effect of escalated dose of bortezomib in re-induction therapy.
- Evaluation of bortezomib in combination with daunorubicin and cytarabine
Eligibility
- Inclusion Criteria:
- Age 18-60 years, inclusive.
- Subjects with a cytopathologically confirmed diagnosis of AML (M0-M7, FAB classification except FAB M3 or t(15;17)), or with refractory anemia with excess of blasts (RAEB) or refractory anemia with excess of blasts in transformation (RAEB-t) with an IPSS score of >= 1.5 or patients with therapy-related AML/RAEB/RAEB-t. Also patients with biphenotypic leukemia may be included.
- Patients who have experienced a previous CR of at least 3 months duration and with a treatment free interval since last treatment of at least 3 months
- WHO performance status <= 2 (see appendix E)
- Written informed consent
- Exclusion Criteria:
- Patients with primary refractory disease or who have relapsed within 3 months after obtaining a first CR
- Anti-leukemia treatment within the last 3 months
- Impaired hepatic or renal function as defined by:
a. ALT and/or AST > 3 x Upper Limit of Normal (ULN), or
b. Bilirubin > 3 x ULN, or
c. Serum creatinin > 3 x ULN (after adequate hydration), or (unless these are most likely caused by AML organ infiltration)- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, infection, hypertension, etcetera), or
- Cardiac dysfunction as defined by:
a. Myocardial infarction within the last 6 months of study entry, or
b. Reduced left ventricular function with an ejection fraction < 50% as measured by MUGA scan or echocardiogram (another method for measuring cardiac function is acceptable), or
c. Unstable angina, or
d. Unstable cardiac arrhythmias- Pregnant or lactating females
- Unwillingness or not capable to use effective means of birth control
Participating Sites
Site
1 results
Order by
Accrual rate
Activation date
NL-Rotterdam-ERASMUSMC
2
