HOVON HO57
Archived
Main info
- Identifier:
- HOVON 57 MM
- Sponsor:
- HOVON
- Included patients:
-
24
- Active sites:
-
10(of 10)
- Title:
A randomized phase III study of i.v. zoledronate (administered for 12 versus 36 months) as an adjunct to standard therapies in the treatment of multiple myeloma.
Timeline
Scheduled
Actual
2004
19 Apr
Activated
2015
31 Oct
Archived
Flow
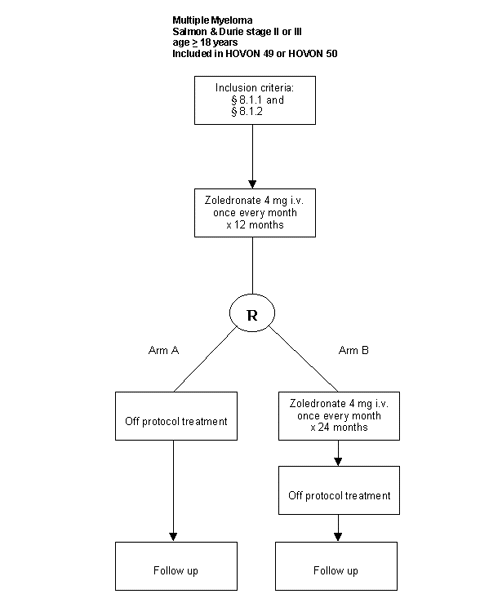
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Patients with a confirmed diagnosis of multiple myeloma stage II or III according to the Salmon & Durie criteria;
- Patients with at least one osteolytic bone lesion on conventional radiographs (plain film);
- Inclusion in HOVON 49 or HOVON 50 trial;
- Inclusion in HOVON 57 at the same time as inclusion in HOVON 49 or HOVON 50;
- Date of inclusion in HOVON 57 trial before date start chemotherapy HOVON 49 or HOVON 50;
- Age >= 18 years;
- WHO performance status 0-3;
- Negative pregnancy test at inclusion if applicable;
- Written informed consent.
Patient Eligibility Criteria for randomization:
- Patients who received zoledronate infusions for 12 months from registration are eligible for randomization if:
Evaluation of radiography and blood parameters has been completed according to protocol.
- Exclusion Criteria:
- Treatment with bisphosphonates at any time during the 12 months prior to registration. Exception: patients may have received up to 3 doses of a bisphosphonate for hypercalcaemia provided this has been administered >= 14 days prior to registration;
- Corrected (adjusted for serum albumin) serum calcium < 2.00 mmol/l or > 2.80 mmol/l;
- Serum creatinin > 265 umol/l;
- Total bilirubin > 30 umol/l;
- Patients unwilling or unable to comply with protocol;
- Severe cardiac dysfunction (NYHA classification III-IV) ;
- Patients with clinically significant hypersensitivity to zoledronic acid or other bisphosphonates;
- Patients who are not willing or capable to use adequate contraception during the therapy (all men, all pre-menopausal women);
- Lactating patients if applicable.
Exclusion criteria for randomisation:
- Development of TIH;
- Corrected (adjusted for serum albumin) serum calcium < 2.00 mmol/l or > 2.80 mmol/l;
Serum creatinin > 265 umol/l;
Total bilirubin > 30 umol/l;
Patients unwilling or unable to comply with protocol;
Severe cardiac dysfunction (NYHA classification III-IV) ;
Patients with clinically significant hypersensitivity to zoledronic acid or other bisphosphonates;
Excessive toxicity;
Progressive disease or relapse from CR.
Participating Sites
Site
10 results
Order by
Accrual rate
Activation date
NL-Rotterdam-ERASMUSMC
11
NL-Amsterdam-VUMC
10
NL-Rotterdam-EMCDANIEL
1
NL-Rotterdam-MAASSTADZIEKENHUIS
1
NL-Amsterdam-OLVG
1
NL-Dirksland-VANWEELBETHESDA
NL-Den Haag-HAGA
NL-Amsterdam-AVL
NL-Amsterdam-AMC
NL-Leiden-LUMC
