About HOVON
Welcome to the website of the HOVON (Stichting Hemato-Oncologie voor Volwassenen Nederland).
HOVON is a foundation with its core business in conducting clinical trials, biomarker and translational studies and developing disease-specific guidelines in the field of hematologic disease. HOVON brings together clinicians and (clinical and basic) scientists and functions as an umbrella organization for all hematologic centers located in the Netherlands and Belgium.
The HOVON office is located at the Erasmus Medical Center in Rotterdam.
Objective & policy
HOVON was founded in 1985 and represents an organization committed to foster progress in the quality of management and outcome of patients with hematologic diseases. It is a registered non-profit foundation.
We believe that the best way of achieving these goals is to establish and conduct clinical trials and related translational studies. It is considered vital to promote the delivery of optimal care to patients by establishing and managing networks of treating physicians in various hospitals.
Since the foundation of HOVON, it is acknowledged that the extension to other countries is vital in establishing impactful clinical trials. In addition to the Netherlands, the HOVON network is therefore expanded to also include Belgium and Luxembourg. Moreover, HOVON has established a collaborative relationship with many other international centers and collaborative groups in conducting clinical trials.
The HOVON has set-up three types of working groups focusing on (A) specific diseases (disease-specific working groups), (B) specific treatment modalities (modality working groups) and (C) specific technical diagnostics and monitoring (technical working groups). Within these groups, novel ideas, studies, clinical trials and guidelines are developed. The guidelines are written by the disease-specific working groups under auspices of the Dutch Hematology Organization (Nederlandse Vereniging voor Hematologie (NVvH). Each disease-specific working group has patient representatives.
The current disease-specific working groups are:
- Benign Hematology, covering non-maligned hematological disorders, such as hemoglobinopathies, auto-immune blood disorders and transfusion medicine
- Chronic Lymphocytic Leukemia (CLL), covering CLL, and leukemic B and T cell diseases such as hairy cell leukemia, large granular lymphocyte leukemia and T-PLL
- Chronic Myeloid Leukemia / Myeloproliferative neoplasms (CML/MPN), covering polycythemia vera, essential thrombocytosis, myelofibrosis and mastocytosis
- Leukemia, covering Myelodysplastic Syndrome (MDS), Acute myeloid leukemia (AML) and Acute lymphocytic leukemia (ALL)
- Lymphoma, covering all lymphoma subtypes like Waldenstroms Macroglobulinemia
- Multiple myeloma (MM), also covering related plasmacell disorders including amyloidosis
The current treatment-modality working groups are:
- Immune Effector Cell (IEC)
- Lunenburg Lymphoma Phase 1 / II Consortium (LLPC)
- Stem cell transplantation (SCT)
- Supportive care
The current technical working groups are:
- HOVON Pathology Facility and Biobank (HOP)
- Imaging working group
- Molecular diagnostics working group including Cyogenetics (MODHEM)
The HOVON has an office in which the following operations are assembled:
- Finance & Legal
- Clinical Operations including secretary, project management, central data management, regulatory, monitoring, IT, pharmacovigilance, QA
- Statistics
Organization of HOVON
The structure of the HOVON foundation appears in the following organization chart:
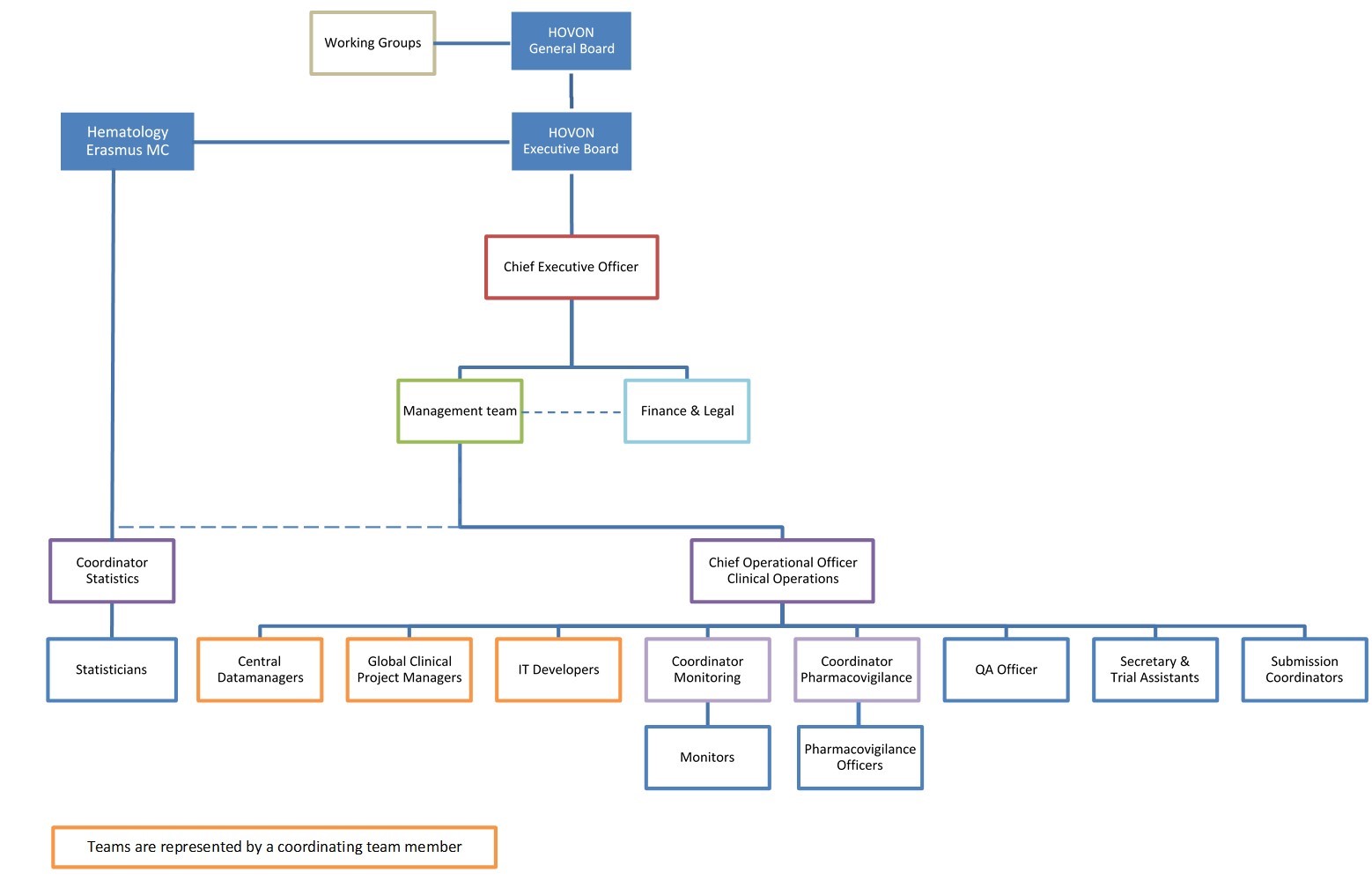
HOVON has a General Board, that elects the Executive Board. The General Board has at least one representative from the hematology departments of the university hospitals in the Netherlands, two general and/or University hospitals in Belgium, six representatives from the general hospitals in the Netherlands and the chairs of the working groups. The Executive Board consists of a chair, a vice chair, a secretary, a treasurer and a general hospital representative.
The day-to-day management of HOVON is delegated to the CEO of HOVON.
The Executive Board members (2024)
The executive board exists out of:
- Representatives of all working groups
- Representatives of echelon B, C and D
- Representatives of academic hospitals
- Representative of Belgium sites
Chair
Prof. Dr. A.P. Kater (AmsterdamUMC , Amsterdam)
Vice chair
Prof. Dr. M.H.G.P. Raaijmakers (Erasmus MC, Rotterdam)
Treasurer
Prof. Dr. G.A. Huls (UMCG, Groningen)
Secretary
Dr. N.P.M. Schaap (UMCN, Nijmegen)
Representant of non-academical hospitals
Dr. S. Kersting (Hagaziekenhuis, Den Haag)
General Board Members
On behalf of the working groups
- Prof. Dr. J.J. Zwaginga (LUMC, Leiden; Echelon A; Chair Working group Benigne Hematology)
- Dr. M.D. Levin (ASZ, Dordrecht; Echelon C-HIC&C-SCT; Vice Chair Working Group CLL)
- Dr. P. te Boekhorst (ErasmusMC, Rotterdam; Echelon A; Chair CML/MPN Working group)
- Dr. M. Chamuleau (AmsterdamUMC, Amsterdam; Echelon A; Chair Working Group Lymphoma)
- Prof. Dr. S. Zweegman (AmsterdamUMC, Amsterdam; Echelon A; Chair MM Working Group)
- Dr. T. van Meerten (UMCG, Groningen; Echelon A; Chair Working Group IEC)
- Prof. Dr. M.J. Kersten (AmsterdamUMC, Amsterdam; Echelon A; Chair Working Group LLPC; Vice-Chair Working Group IEC)
- Dr. A. Broers (ErasmusMC, Rotterdam; Echelon A; Vice-chair Working Group SCT)
- Dr. J. Heijmans (AmsterdamUMC, Amsterdam; Echelon A; Chair Working Group Supportive care)
- Dr. A. Diepstra, (UMCG; Echelon A; Chair HOVON Pathology Facility and Biobank)
- Prof. Dr. J. Zijlstra (AmsterdamUMC, Amsterdam; Echelon A; Chair Working Group Imaging)
- Dr. B.A. van der Reijden (UMCN, Nijmegen; Echelon A; Chair Working Group Moleculair Diagnostics)
- Dr. B. Beverloo (ErasmusMC; Echelon A; Chair Working Group Cytogenetics)
On behalf of echelon B, C, D, Belgium
- Dr. S. Kersting (Hage ziekenhuis, Den Haag; Echelon B)
- Dr. O Visser (Isala Ziekenhuis, Zwolle; Echelon B)
- Dr. M. Hoogendoorn (MCL, Leeuwarden; Echelon C-HIC&C-SCT)
- Dr. G.J. Timmers (Ziekenhuis Amstelland, Amstelveen; Echelon D)
- Dr. I. Verpoorte (Ziekenhuis Rivierenland, Tiel; Echelon D)
- Prof. Dr. P. Vandenberghe (UZLeuven, Leuven, Belgium)
- Dr. M.C. Vekemans (UC Louvain, Louvain-La-Neuve, Belgium)
On behalf of academic hospitals
- Prof. Dr. A.P. Kater (AmsterdamUMC , Amsterdam; Echelon A; Chair Working Group CLL)
- Prof. Dr. M.H.G.P. Raaijmakers (Erasmus MC, Rotterdam; Echelon A; Vice Chair working Group Leukemia)
- Prof. Dr. J.H. Veelken (LUMC, Leiden; Echelon A)
- Dr. M. van der Poel (MUMC, Maastricht; Echelon A)
- Prof. Dr. J.H.E. Kuball (UMCU, Utrecht; Echelon A)
- Prof. Dr. G.A. Huls (UMCG, Groningen; Echelon A; Chair Working Group Leukemia)
CEO
Dr. M.C. Breems- de Ridder
