Pharmacovigilance
SAE form
All trials have trial specific SAE forms. These can be found at the download section of the trial. Please find below a general version of the SAE instructions that serve as a guide to fill out the forms.
SAE Instructions
We also set up an SAE Reporting doc that shows which items are required on an Initial, Follow up and Final SAE report respectively.
SAE Reporting doc
SAE evaluation
Based on a minimum number of data, it can be concluded from an SAE if it qualifies as a Suspected Unexpected Serious Adverse Reaction (SUSAR). To make clear which items are important to everyone, there is a simple tool (SAE Reporting) made for the persons who report SAEs. This document contains the items which should at least be filled out on a first report of a SAE. (But also what we expect a Follow-up and a Final SAE.)
Mostly the SAE evaluation is performed by the "safety monitors" of the HOVON Pharmacovigilance team. However, in exceptional cases it is sometimes decided that this task shall be performed by the principal investigator.
An SAE qualifies as SUSAR if:
- The (S)AE-term is seen a considerable relationship, compared to one of the study drugs as the Investigational Medicinal Product (IMP) were appointed and
- The term is not named in the Investigators Brochure (IB) of the related IMP.
The relationship between the SAE and the medication is to be assessed by the site principal investigator. If the investigator sees no connection, the safety monitor (in consultation with the principal investigator) can determine that there is a relationship. The reverse situation is not possible. The Pharmacovigilance team uses an internationally applied tool:
SAE data management
Based on the SAE report (Initial / Follow-up / Final) that we receive, we look at whether information is missing and if the information on the report is consistent with the protocol and the previously reported information. If anything is not clear or if there is an inconsistency is detected, we send questions (queries) to the site which reported the SAE.
A distinction is made between:
- Urgent queries: Within 2 days we expect an answer. These queries will be about missing or unclear parts that are important and needed to carry out the SAE-evaluation.
- Non-ugent queries: Within 14 days we expect an answer. This may affect all issues that are important to have a clear picture of the SAE.
Answering the queries:
The answers to the e-mail with queries can preferably be done by emailing us a revised SAE, including our questions. You can best send the package (revised SAE incl. our queries) to the contact details listed at the header of the SAE report.
SUSAR reporting
By current laws and regulations, SUSAR’s need to be reported to a number of parties, namely:
- The judging Ethics Committee (EC)
- The Competent authority (CA)
- The European Medicine Agency (EMA / http://www.ema.europa.eu ) in the EudraVigilance Database.
- All local researchers in the study where the SUSAR occurs
The HOVON Pharmacovigilance team will also report each SUSAR to:
- The principal investigator,
- The principal investigator of other studies with that same IMP in his / her study. (We do this because HOVON as sponsor is obliged to report this SUSAR’s to the EC / CA of other studies if they are relevant for that study. The relevance should be assessed by the PI of that study).
Local Coordinator SAE
For each trial, we request at the local contact form, the Local Coordinator SAE.
This person will receive a copy of all SAE queries and SUSAR’s, which the HOVON Pharmacovigilance team will send to the site principal investigator(s) of the hospitals, so that he / she can ensure a timely and correct processing.
If you also want to be a local SAE coordinator in your hospital, please sent an e-mail to the HOVON Pharmacovigilance team. We will than process your request (this usually takes 2 working days). After your request is processed you, but also the site principal investigator, will receive a message that you are included in the distribution list.
Frequently Asked Queries
The Frequently Asked Questions (FAQ) document has been developed to diminish the amount of questions about the SAEs.
Please see the link to the SD FAQ document below.
Pharmacovigilance team FAQ doc
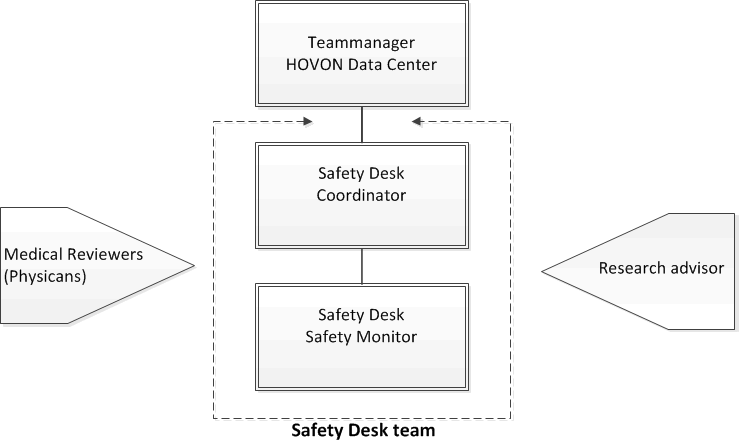
HOVON Pharmacovigilance team set-up
The organizational set-up is shown below: