Trial Close-out
Each HOVON trial will have the timelines shared on this website at section Trials. Both scheduled and actual timelines are shown. These include also Close-out in progress / Last patient out and Archived.
The Last patient out will differ per site, based upon the last patient that was included for that site. We have the following guidelines we adhere to in assessing the Follow up duration (measured from the date of registration):
- Phase I studies: duration of follow up from end of treatment 6 months
- Phase II studies: duration of follow up from inclusion 5 years or shorter in line with the expected response duration
- Phase III studies: duration of follow up from inclusion 5 years preferably (with a maximum of 10 years)
The above general timelines do not take into account trials with maintenance, for these trials please refer to the protocol.
Pharmacy Close-out
For HOVON sponsored sites a pharmacy close-out can be performed if all enrolled patients of a particular site are off protocol treatment.
The participating pharmacist will be contacted by the Global Clinical Project Manager (CPM) of the trial once the pharmacy close-out can take place.
He / she will provide information on the steps to take in order to close down the trial at the pharmacy.
Because patients are still in follow-up the second part of the site close-out will take place when the last patient out date for the site has been reached.
When co-sponsors participate in a trial site close-out will be performed according to the national policy.
Site Close-out
HOVON will try to perform the site close-out as soon as possible, once the last patient out date has been reached and all data has been send in and no queries left open.
The participating site will be contacted by the Global CPM of the trial once the close-out can take place. He / she will provide information on the next steps to take in order to fully close down the trial at the site.
If you think your site is sooner ready for a close-out, please let the Global CPM know (contact details can be found at the Participating parties section of the trial – after log in).
Please do not forget to check if you requested reimbursement for all patients enrolled at your site.
Site Archived
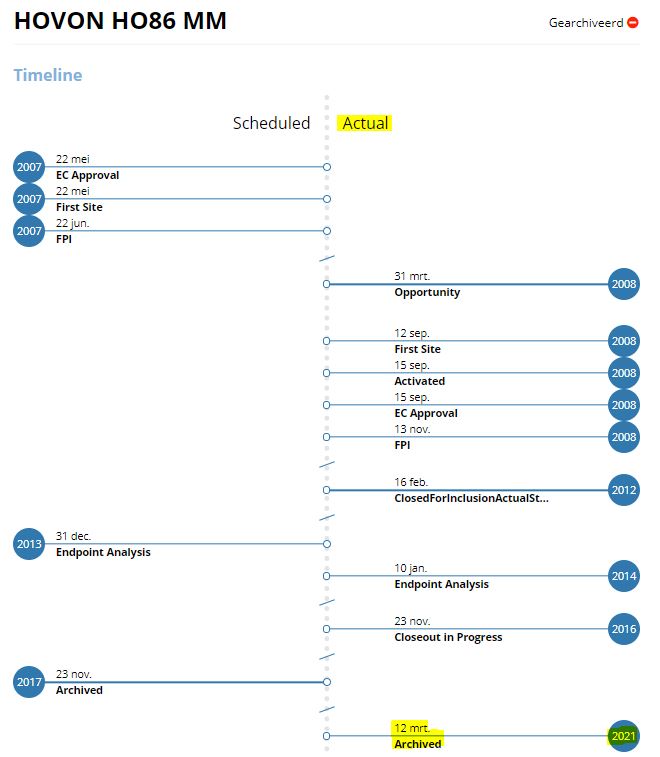
Per trial (in the trial section) you can see the status of the trial. If it is stated as Archived, we also have entered the Actual Archived date (in the study timeline overview). This is the date on which you are allowed to archive the trial at your site as well, unless you have received information from the Global CPM that indicates another (earlier) archive date.
Please note that the archive date is always the date on which you are allowed to send out the Investigator Site File to the (external) archive, this differs from the destruction date! The destruction date for participating sites is 25 years after archive date. After this destruction date you are allowed to destruct the study documents.
Source documents (i.e. medical records) of patients should be retained in accordance with national law.
If in doubt with regards to the archive and/or destruction date, please contact the Global CPM of the trial.
Below is an example of a study with an Actual Archived date (note: never use the Scheduled Archived date, this is not a fixed date and can be still adjusted)