HOVON HO68
Main info
- Identifier:
- HOVON 68 CLL
- Sponsor:
- HOVON
- Included patients:
-
281
- Active sites:
-
0(of 58)58 sites are pending
- Title:
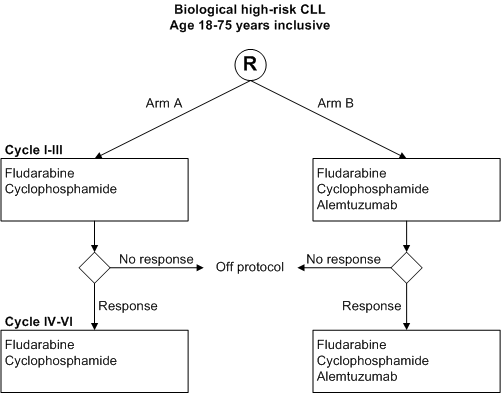
A randomized phase III study in previously untreated patients with biological high-risk CLL: Fludarabine + cyclophosphamide (FC) versus FC + low-dose alemtuzumab.
Timeline
News
As of 12NOV2010 the Ho68 CLL study is closed for inclusion of new patients.
Reason for closure is the publication of results of the CLL8 study. The Writing Committee no longer considers it feasible to randomise patients to FC chemotherapy without monoclonal antibody.
A letter of closure will be sent to all participating sites.
Flow
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Determination of the efficacy and safety of oral fludarabine and cyclophosphamide plus concomitant s.c. alemtuzumab compared to fludarabine and cyclophosphamide alone in terms of progression free survival, event free survival, clinical, flow cytometric and molecular response rates, overall survival and disease free survival.
Eligibility
- Inclusion Criteria:
- Biological high-risk CLL*;
- Patients with symptomatic** stage A, symptomatic** stage B or stage C (see appendix B);
- Age 18-75 years inclusive;
- Written informed consent. * Biological high risk is defined as: >= 98 % homology of IgVH genes with germ-line sequences and/or mutated CLL with usage of VH3-12 and/or FISH with 17p deletions and/or 11q deletions and/or trisomy 12.** Symptomatic CLL is defined according to the NCI criteria for active disease (Cheson et al. 1996, see appendix A).
- Exclusion Criteria:
- WHO performance status >= 3 (see appendix E), unless related to CLL;
- Intolerance of exogenous protein administration;
- Severe cardiac dysfunction (NYHA classification III-IV, see appendix F);
- Significant renal dysfunction (serum creatinine >= 150 µmol/l or creatinine clearance < 30 ml/min);
- Significant hepatic dysfunction (total bilirubin or transaminases > 2 times ULN), unless related to CLL;
- Suspected or documented CNS involvement by CLL;
- Known seropositivity of HIV, Hepatitis B and C
- Active, uncontrolled infections;
- Uncontrolled asthma or allergy requiring systemic steroid treatment;
- Previously treated with chemotherapy, radiotherapy or immunotherapy for CLL;
- History of active cancer during the past 5 years, except non-melanoma skin cancer or stage 0 cervical carcinoma;
- Clinically significant auto-immune hemolytic anemia (AIHA);
- Female patients who are pregnant or nursing;
- Male and female patients of reproductive potential who are not practicing effective means of contraception,these include oral contraceptives, intrauterine device, depot injection of gestagen, subdermal implantation, hormonal vaginal ring and transdermal depot plaster. These methods must be applied for the entire protocol treatment period, and for patients treated with alemtuzumab until at least 6 months after the end of alemtuzumab administration.
