HOVON Pathology Facility and Biobank (HOP)
Composition
Chair
Dr. A. Diepstra (UMCG, Groningen)
Members
Li-Ping Fu – Daily coordinator (AmsterdamUMC, Amsterdam)
Dr. L. Koens (AmsterdamUMC, Amsterdam)
Dr. W.B.C. Stevens (RadboudUMC, Nijmegen)
Dr. M. Nijland (UMCG, Groningen)
Key objectives of working group
The key objectives of this working group are:
- Support of all pathology-related activities for clinical trials by HOVON
- Protocol design and preparation
- Pathology review
- Biobanking
- Tissue-based research
- Serving as central contact on all pathology-related quesions for all participants
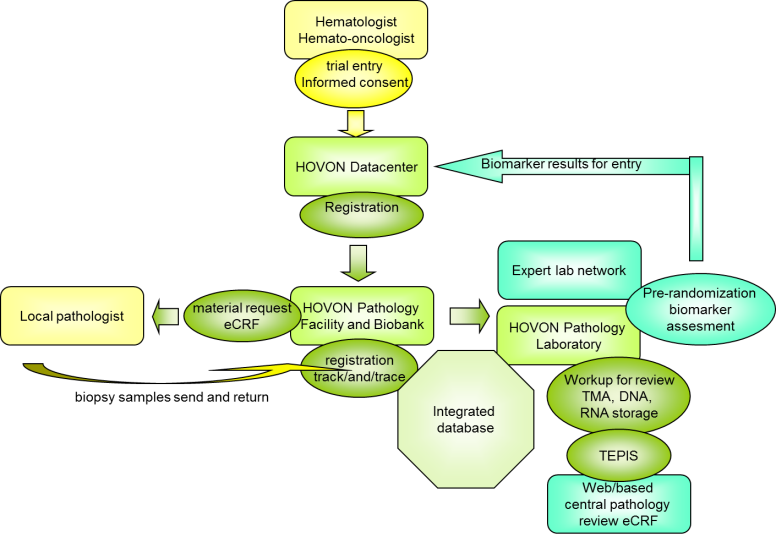
The logistics of pathology review for HOVON clinical trials is based on a central registration database with a track-and-trace module to ensure availability and traceability of material at all times. Material is received from local pathology laboratories and processed for pathology review and biobank storage for side studies in the form of tissue micro-array (TMA) and material for subsequent DNA and RNA isolation. After processing, the remaining tissue is sent back promptly, aimed within 3 months.
The workflow, based on LDOT is designed for a speedy review process, which is performed during the course of a clinical trial, making the pathology review data available immediately after completion of the trial. Review data can be made also available for planned interim analyzes and upon request of the appropriate parties from HOVON-DB, HDC or LWG. Centralization makes the review process cost-effective. HOP is designed in such a way to minimize turn-around times enabling implementation of pre-randomization assessments by the HOP laboratory or partner laboratories for (future) biomarker-based clinical trials.
Side studies can be conducted efficiently, because the material is stored centrally and informed consent information is fully recorded within the system. The material is available for all research groups in the Netherlands on the basis of project proposals, which are reviewed by the steering committee of HOP and approved by the Lymphoma Working Group.
All biomarker data collected from biopsies are stored in the HOP research database, which is designed for compatibility with the HOVON Data Center (HDC) clinical database and databases of the other HOVON technical working groups, including the HOVON Imaging Group.
Pathology review of clinical trials is performed in principle by two pathologists for each trial, preferably an academic and non-academic pathologist. As part of the preparation for clinical trials, HOP is responsible for informing and recruiting hematopathologists in the Netherlands for central pathology review. The final assignment to a specific trial is made by HOP and the Principal Investigator(s) of the trial on the basis of interest and expertise in the topics covered by a specific trial or lymphoma type. Assignment is approved by the LWG. We aim for a balanced regional contribution and involvement of many hematopathologists in the Netherlands.
Work Flow and information for local pathology laboratories and submitters
When a patient is enrolled in a HOVON lymphoma trial, a notification is automatically send out to HOP so the patient can be included in the HOP Database System. As soon as informed consent for pathology review is registered, HOP requests biopsy material from the local pathology laboratory.
During the trial, the material is requested in a real time manner and processed immediately for review and storage. If part of the trial protocol (for bio-marker-based trials), pre-randomization assays can be performed in the HOP laboratory or in one of the partner laboratories. After processing, the material is sent back to the original pathology laboratory.
All material is processed and stored according to a standardized protocol under the guidelines of the HOVON Biobanking Procedures unless otherwise stated in the Trial Study Protocol.
Standard protocol for processing pathology material in the context of HOVON clinical trials:
The following is requested:
- Formalin-fixed / paraffin-embedded tissue block from a representative lymph node or extranodal lymphoma localization
- Copy of the accompanying (local) pathology report, including any assessments by regional panels and / or consultations.
- If only a limited amount of material is available (eg in case of endoscopic biopsies or thick needle biopsies), it is preferable to send the immunohistochemical stains from the original pathology laboratory immediately. These will be returned after the review process.
Processing material for review:
- Next to HE slides, immunohistochemical and/or molecular assays are performed only in the context of the inclusion criteria for the specific trial (eg specific lymphoma diagnosis, CD20, CD30, translocation FISH)
- Additional immunohistochemical and/or molecular assays in the context of planned research studies that are part of the Trial Study Protocol are also performed in this phase.
Processing for HOP Biobanking
See also:
Guidelines for Pathology review within HOP (Dutch only)
- 2x 0.6-1.0 mm FFPE cores for tissue microarray
- ± 5 FFPE scrolls for storage for DNA isolation (-80°)
- ± 5 FFPE scrolls for storage for RNA isolation (-80°)
Fee for sending material
Pathology review is included in the budget of each trial, separated for the central review and for covering costs by local pathology departments and laboratories. For each enrolled patient, a lump sum will be paid by HOVON as reimbursement for the costs incurred to the including hospitals, generally through the local hematology department. The costs of local pathology departments are included in this lump sum and these parties should claim their expenses at their local receiving department. Costs cannot be claimed separately or additionally from HOVON or HOP. Further information with regards to reimbursement are referred to Hovoninvoice@amsterdamumc.nl
Overview Archive HOVON Pathology Facility and Biobank (HOP)
Central pathology review is an integral part of HOVON lymphoma trials. In addition to confirmation of the inclusion diagnosis as quality assurance of the clinical trial as such, the secondary aim of this review is to secure material for tissue-based laboratory research studies both included in the trial protocol and subsequently proposed (unplanned) studies. To this end, tissue microarrays (TMAs) are constructed for all cases with sufficient available formalin-fixed/paraffin-embedded (FFPE) material. FFPE scrolls in Eppendorf tubes for isolation of DNA and/or RNA and/or other relevant laboratory techniques are stored at -20˚. If limited FFPE material is available, a prioritization is applied based on expected research interests.
The attached overview (link below) gives information on the HOP Biobank archived materials per trial. Techniques that have been applied to the available material in the context of concluded side studies is indicated. Requests for the use of materials from the HOP Biobank should be made using the HOVON standard form and will be issued by HOP after approval by the Lymphoma Working Group. HOP encourages and supports the re-use of research data. Contact details of responsible researchers of previous side studies, of which the (laboratory) techniques used are listed, can be requested via HOP and/or the principle Investigators of the clinical trial.
Used abbreviations:
| IHC | immunohistochemistry (single slides, TMA) |
| FISH | fluorescent in situ hybridization for translocation detection (single slide, TMA) |
| NGS | next-generation sequencing (targeted, whole exome, whole genome sequencing) |
| GEP | gene expression profiling |
| AI | morphological analysis using artificial intelligence |
| VECTRA | multiplex immunohistochemistry (microenvironment analysis) |
An overview on HOP trials is visible at the bottom of this page after you have logged in.
Please note that the HOVON policy with regards to requesting material/data can be found at About HOVON > HOVON protected policy documents (HOVON template to request data from HOVON trials)
Contact
HOVON Pathology Facility (HOP)
AmsterdamUMC/VU University Medical Center
Department of Pathology, ZH 1E20
De Boelelaan 1117
1081 HV Amsterdam
The Netherlands
e-mail: hop@vumc.nl
