HOVON HO102 AML
Main info
- Identifier:
- HO102 AML
- Sponsor:
- HOVON
- Working group party:
- Leukemia
- Age:
- 18-65
- Stage:
- 1st Line
- Echelon:
- Limited Site Selection
- Included patients:
-
890(of 920)
- Active sites:
-
45(of 50)
- Title:
Randomized study with a run-in feasibility phase to assess the added value of Clofarabine in combination with standard remission-induction chemotherapy in patients aged 18-65 years with previously untreated acute myeloid leukemia (AML) or myelodysplasia (MDS) (RAEB with IPSS ≥ 1.5)
Timeline
News
28Sep2023: Global LPLV (Last Patient Last Visit) is reached. Sites will be informed about close-out and end of trial form.
28Sep2013: Closure of study - The study is closed for further inclusion from 28SEP13 onwards.
New documents:
- New WMO insurance certificate for NL + BE sites is available (runs till 01DEC14) (05NOV13)
- New KWF approval document (with expansion of patient numbers) (09JUL13)
- New WMO insurance certificate for NL sites is available (runs till 01DEC13) (29JAN13)
12OCT12: New documents regarding AM9
- ICF BM sampling NL
- CA + EC approval portfolio updated
02OCT12: Updated version of the FAQ sheet
03MAY12: New CRF related documents:
- CRF (clean version as well as tracked changes version)
- DLT form
- Registration and randomisation form (no changes in content, only version date changed)
- CRF instructions (clean version as well as tracked changes version)
24APR12: New documents regarding AM7
- Protocol: clean version + tracked changes + summary of changes
- EC approvals portfolio
- CA approvals portfolio
24OCT10:New version of Pharmacy information letter (with lower maximum amount of Clofarabine kits to order).
08OCT10: New version of Pharmacy information letter (with other contact details regarding PENN).
06SEP10: New version of Pharmacy information letter (with more additions on guidelines reordering Clofarabine).
21JUL10: New version of Pharmacy information letter (with addition on guidelines reordering Clofarabine).
15JUL10: New version of the drug accountability form (only given example on page 2 has been changed).
06MAY10: NEW SAE form, because fax number on first page was incorrect, please use HO102 SAE report_06MAY10
16APR10: The Frequently Asked Questions has been updated.
08APR10: The Frequently Asked Questions has been updated.
05MRT10: The CRFs have been updated (current version 05MRT10), because of erroneous and missing information on form 16.
19NOV10: Please be aware: the dosage of Clofarabine has been reduced for investigational arm to 10 mg/m2 (as of 19NOV10)
As of 14DEC10 we have started PART B of this study.
Flow
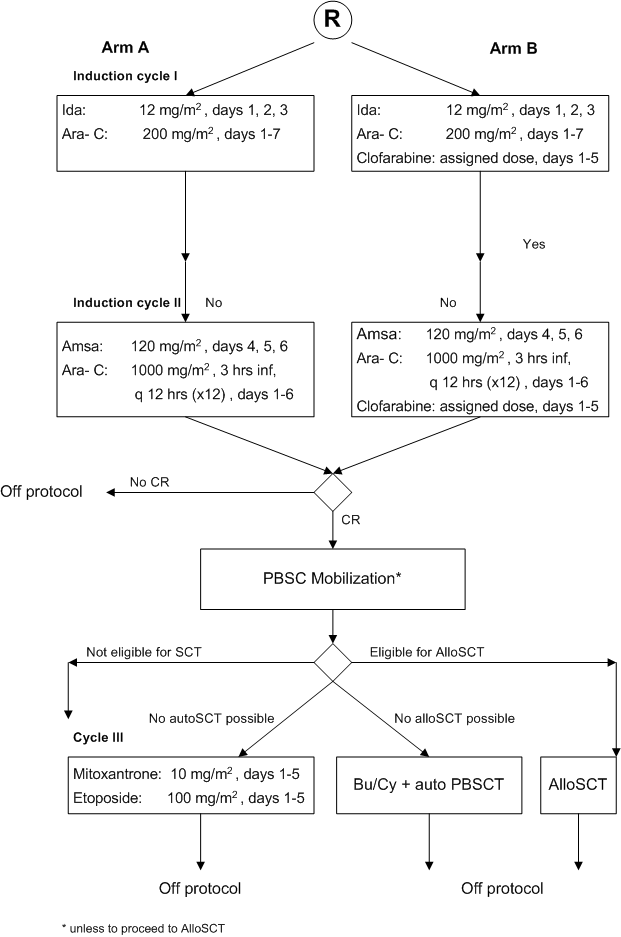
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Not any more
- Objectives:
Primary objectives
For part A of the study:- To determine the feasibility of Clofarabine when given at three possible dose levels together with standard induction cycles I and II in patients with AML/ RAEB with IPSS≥1.5 in a prospective comparison to standard induction cycles I and II without Clofarabine.
For part B of the study:
- To evaluate the effect of Clofarabine at the selected feasible dose level when combined with remission induction chemotherapy cycles I and II as regards clinical outcome (“event-free survival”) in comparison to remission induction cycles I and II with no addition of Clofarabine in a phase III study.
Secondary objectives
For part A of the study:- To investigate the clinical efficacy of Clofarabine in combination with remission induction chemotherapy cycles I and II with regard to complete remission rate at different dose levels of Clofarabine.
For part B of the study:
- To investigate the clinical efficacy of Clofarabine with regard to the complete remission rate, disease free survival (DFS), risk of relapse and overall survival (OS) when combined with remission induction chemotherapy cycles I and II in all patients.
- To investigate the clinical efficacy of Clofarabine when combined with remission induction chemotherapy cycles I and II in molecularly and cytogenetically distinguishable subsets with regard to the complete remission rate, disease free survival (DFS), risk of relapse and overall survival (OS).
- To investigate the tolerance and toxicity of Clofarabine in combination with remission induction chemotherapy cycles I and II.
- To assess the effect of Clofarabine on peripheral CD34 cell numbers for autologous peripheral blood transplantation.
- To determine the prognostic value of molecular markers and gene expression profiles of the leukemia assessed at diagnosis.
- To evaluate the treatment effects according minimal residual disease (MRD) measurements following therapy by standardized sampling of marrow/blood.
- To evaluate the outcome of allogeneic sibling or unrelated donor SCT and autologous SCT in cytogenetically and molecularly defined prognostic subgroups of patients.
Eligibility
- Inclusion Criteria:
- Age 18-65 years, inclusive
- Subjects with
- a cytopathologically confirmed diagnosis of AML according WHO classification (excluding acute promyelocytic leukaemia) or
- a diagnosis of refractory anemia with excess of blasts (RAEB) and IPSS score ≥1.5 or
- patients with therapy-related AML/RAEB or
- patients with biphenotypic leukemia (Appendices A1 and A2).
- Adequate renal and hepatic function tests as indicated by the following laboratory values:
- Serum creatinine ≤1.0 mg/dl (≤ 88.7 micromol/L); if serum creatinine >1.0 mg/dl (>88.7 micromol/L), then the glomerular filtration rate (GFR) must be >60 ml/min/1.73 m2 as calculated by the Modification of Diet in Renal Disease equation where the predicted
GFR (ml/min/1.73 m2) = 186 x (Serum Creatinine in mg/dl)-1.154 x (age in years)-0. 203 x (0.742 if patient is female) x (1.212 if patient is black)
NOTE: if serum creatinine is measured in micromol/L, recalculate it in mg/dl according to the equation: 1 mg/dl = 88.7 micromol/L) and used above mentioned formula.- Serum bilirubin ≤1.5 × upper limit of normal (ULN)
- Aspartate transaminase (AST)/alanine transaminase (ALT) ≤2.5 × ULN
- Alkaline phosphatase ≤ 2.5 × ULN
- WHO performance status 0, 1 or 2 (see Appendix I)
- Written informed consent
- Exclusion Criteria:
- *Acute promyelocytic leukaemia
- Previous treatment for AML or RAEB, except hydroxyurea
- Concurrent history active malignancy in two past years prior to diagnosis except for:
- basal and squamous cell carcinoma of the skin
- in situ carcinoma of the cervix
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, infection, hypertension, pulmonary disease etcetera),
- Cardiac dysfunction as defined by:
- Myocardial infarction within the last 6 months of study entry, or
- Reduced left ventricular function with an ejection fraction < 50% as measured by MUGA scan or echocardiogram (another method for measuring cardiac function is acceptable), or
- Unstable angina, or
- Unstable cardiac arrhythmias
- Pregnant or lactating females
- Unwilling or not capable to use effective means of birth control
Registration Details
Eligible patients should be randomized before start of induction treatment. Patients can be randomized via the Internet via TOP (Trial Online Process; https://www.hdc.hovon.nl/top) or at the HOVON Data Center of the Erasmus MC Rotterdam – location Daniel by phone call: +31.10.7041560 or fax +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET. A logon to TOP can be
requested at the HOVON Data Center for participants.
The following information will be requested at randomization:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Local patient code (optional)
- Sex
- Date of birth
- Date of diagnosis of AML or RAEB
- Date written informed consent
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
