HOVON HO105 NHL
Main info
- Identifier:
- HO105 NHL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- 18-70
- Stage:
- 1st Line
- Echelon:
- Level C-HIC
- Included patients:
-
202(of 202)
- Active sites:
-
28(of 29)
- Title:
Rituximab in Primary Central Nervous system Lymphoma. A randomized HOVON / ALLG intergroup study
Timeline
News
Amendment 5, concerning protocol (17DEC12), ICF template (17DEc12) and ICF template partner (17DEC12) has been approved by the cEC and CA of The Netherlands and must be implemented in Dutch sites from 22FEB2013 on.
Flow
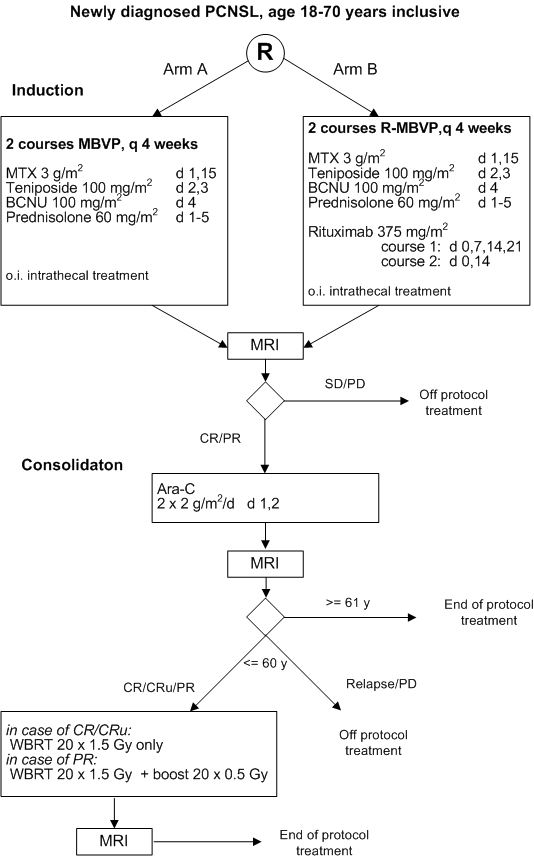
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Not any more
- Objectives:
Primary objective:
- To assess the effect of the addition of rituximab in a standard chemotherapy regime on EFS in newly diagnosed PCNSL
Secondary objective
- To evaluate the effect of the addition of rituximab to a standard chemotherapy regimen with respect to toxicity
Eligibility
- Inclusion Criteria:
- Patients with a histologically confirmed diagnosis of CD20 positive DLBCL based upon a representative histology specimen of brain biopsy according to the WHO classification (see appendix A): OR
- Patients with a diagnosis of PCNSL based on MRI evidence of brain parenchymal lesion showing homogeneous contrast enhancement suspect for lymphoma AND
- Unequivocal morphological and/or immunophenotypical evidence of CSF CD20 + large cell lymphoma
- AND/OR Unequivocal morphological and/or immunophenotypical evidence of CD20 + large cell lymphoma in vitreous fluid OR
- Patients with unequivocal morphological and/or immunophenotypical evidence of CD20 + large cell lymphoma in vitreous fluid AND CSF but without a brain parenchymal lesion
- Age 18-70 years inclusive
- Performance status with or without administration of steroids WHO/ECOG 0 – 3 (see appendix D)
- Written informed consent
- Exclusion Criteria:
- Evidence of systemic lymphoma
- History of intolerance of exogenous protein administration
- Severe cardiac dysfunction (NYHA classification III-IV, appendix G, or LVEF < 45%)
- Congestive heart failure or symptomatic coronary artery disease or cardiac arythmias not well controlled with medication
- Severe pulmonary dysfunction (vital capacity or diffusion capacity < 50% of predicted value)
- Significant hepatic dysfunction (bilirubin or transaminase ≥ 2.5 x upper normal limit) at Screening.
- Significant renal dysfunction (serum creatinine ≥150 μmol/l or clearance < 60 ml/min) at Screening
- Presence of “third space fluid”, such as pleural effusion or ascites
- Prior cranial radiotherapy
- Active uncontrolled infection
- HIV-positivity
- (EBV positive) post-transplant lymphoproliferative disorder
- Untreated hepatitis B infection (inclusion is possible if adequate antiviral medication e.g. lamivudine or alternative is started and continued for the duration of the trial)
- Positive pregnancy test in women of reproductive potential
- Lactating women
- Unable or unwilling to use adequate contraceptive methods (all men, pre-menopausal women) until 12 months after last chemotherapy treatment
- Any psychological, familial, sociological or geographical condition potentially hampering compliance with the study protocol and follow-up schedule
- An active malignancy, that is expected to require treatment with chemotherapy within one year, or results in a life expectancy less than one year.
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- By ALEA; Use goto eCRF button > select the [Patient tab] and click the [Add new patient] button. Complete all items and click the [Submit] button
- By faxing the completed registration/randomization CRF +31 (0)10 704 1028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31 (0)10 704 1560 Monday through Friday, from 09:00 to 17:00 CET
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Patient’s name code
- Gender
- Date of birth
- Date written informed consent
- Specific items patient gives consent for (see ICF)
- Eligibility criteria
- Stratification factors
All eligibility criteria will be checked with a checklist. Patients will be randomized, stratified by center, age (≤ 60 vs ≥ 61 years) and WHO/ECOG performance status (0-1 vs 2-3) with a minimization procedure, ensuring balance within each stratum and overall balance. Each patient will be given a unique patient study number. Patient study number and result of randomization will be given immediately by TOP or phone and confirmed by fax or email.
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
