HOVON HO108 MM
Main info
- Identifier:
- HOVON 108 MM
- Sponsor:
- HOVON
- Included patients:
-
31
- Active sites:
-
7(of 7)
- Title:
A randomized phase II study for evaluation of T cell depleted non-myeloablative allogeneic stem cell transplantation followed by early consolidation with lenalidomide or lenalidomide combined with bortezomib and subsequent DLI for patients with multiple myeloma in progression or relapse following first line therapy.
Timeline
News
Closure of study
The study is closed for further inclusion from 05SEP13 onwards.
New documents:
20MAR13: Upload of HO108 specific IMP documentation. Please use these instead of the commercially available version.
20SEP12: New statement of expenses form (with addition of patient numbers)
22MAR12: Update of following documents regarding amendment 3:
- EC approval portfolio
- CA approval portfolio
- Protocol (clean version + summary of changes)
17JAN12: Declaration form (QOL expenses added)
16JAN12: Update of following documents regarding amendment 2:
- EC approval portfolio
- CA approval portfolio
- Protocol (clean version + summary of changes)
- ICF (clean version + summary of changes)
- CRF (clean version + summary of changes)
10JAN12: Addition of Labmanual (version 3 - 10JAN11)
23NOV11: Addition of declaration form
21NOV11: NEW SAE form, changed fax number, please use HO108_SAE form_21NOV11
26OCT11: NEW SAE form, based on template version 03
Flow
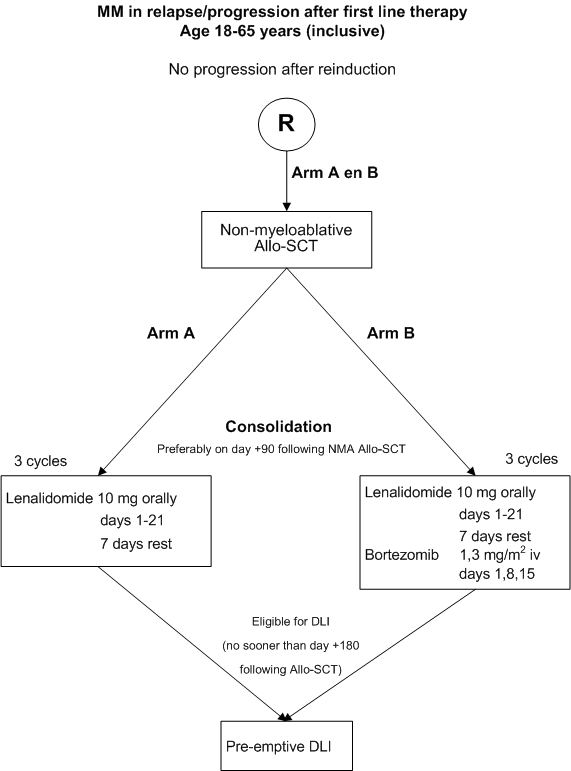
Details
- Phase:
- Prospective randomized Phase II study
- Monitoring Type:
- Objectives:
Assesment of feasibility, toxicity and efficacy of lenalidomide and of lenalidomide combined with bortezomib as early consolidation after T cell depleted non myeloablative Allo-SCT and before DLI; Asssessment of the efficacy of non myeloablative Allo-SCT as treatment of relapsed multiple myeloma in terms of complete remission rate, overall and progression free survival.
Eligibility
- Inclusion Criteria:
- Patients with multiple myeloma with a first relapse after first line therapy;
- Relapsed patients have received reinduction therapy before entering this trial;
- At least a PR after reinduction treatment
- 18-65 years, inclusive;
- HLA- identical sibling or unrelated donor completely matched (10/10)
- WHO-performance status 0-2;
- Written informed consent.
- Exclusion Criteria:
- Previous Allo-SCT;
- Severe pulmonary dysfunction (CTCAE grade III-IV, see appendix D);
- Severe neurological or psychiatric disease;
- Patients with neuropathy, CTC grade 3 or higher;
- Significant hepatic dysfunction (serum bilirubin or transaminases ≥ 3 times upper limit of normal);
- Significant renal dysfunction (creatinine clearance < 30 ml/min after rehydration);
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, infection, hypertension, cancer, etc.);
- History of active malignancy during the past 5 years with the exception of basal carcinoma of the skin or or carcinoma “in situ” of the cervix or breast;
- Patient known to be HIV-positive;
- Patients with brain disease with the exception of those patients whose brain disease has been treated with either radiotherapy or surgery and remains asymptomatic, with no active brain disease, as shown by CT scan or MRI, for at least 6 months;
- The development of erythema nodosum if characterized by a desquamating rash while taking thalidomide, lenalidomide or borium;
- Pregnant or breast-feeding female patients. Negative pregnancy test at study is mandatory for female patients of childbearing potential,
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule;
- Severe cardiac dysfunction (NYHA classification II-IV, see appendix E).
