HOVON HO115 SCT
Archived
Main info
- Identifier:
- HOVON 115 double UBCT
- Sponsor:
- HOVON
- Working group party:
- SCT & Supportive care
- Age:
- 18-70
- Stage:
- 1st Line
- Echelon:
- Level A
- Included patients:
-
70(of 70)
- Active sites:
-
7(of 7)
- Title:
Double umbilical cord blood transplantation in high-risk hematological patients. A phase II study focusing on the mechanism of graft predominance
Timeline
Scheduled
Actual
2012
31 Jan
Opportunity
2012
10 Jul
Activated
2012
10 Jul
EC Approval
2012
20 Aug
First Site
2012
22 Aug
FPI
2015
18 Jun
ClosedForInclusionActualStart
2020
28 Jan
CloseoutInProgressLastPtOutActualStart
2020
18 Jun
CloseoutInProgressLastPtOutScheduledStart
2021
30 Mar
Archived
Flow
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
- To study the presence of an alloreactive immune response of CD4+ T-cells of the predominant CBU, directed against the non-engrafting CBU as a causative mechanism in CBU predominance.
- To assess engraftment and engraftment kinetics;
- to evaluate immune reconstitution, acute and chronic GVHD, chimerism, toxicity, progression-free survival and overall survival after double unit UCBT.
Eligibility
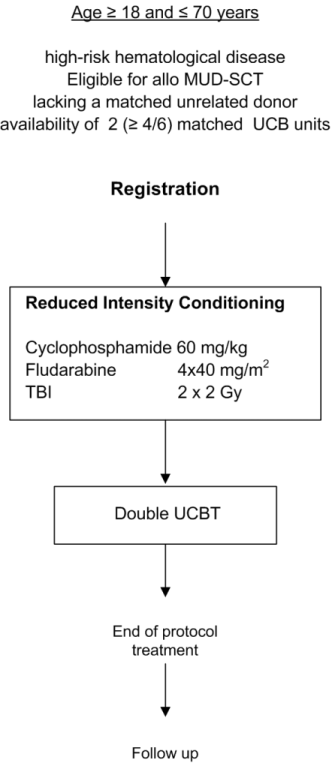
- Inclusion Criteria:
- Age 18-70 years inclusive
- Diagnosis of poor-risk hematological malignancy or (V)SAA relapsing after or failing immunosuppressive therapy and meeting the criteria for a MUD allo SCT
- Lacking a sufficiently matched volunteer unrelated donor or lacking such a donor within the required time period of ≤ 2 months in case of
urgently needed alloSCT
- Availability of 2 (≥4/6) matched UCB grafts with a total nuclear cell count > 4 x 107/kg (see paragraph 8.2).
- WHO performance status 0-2
- Written informed consent
- Exclusion Criteria:
- Bilirubin and/or transaminases > 2.5 x normal value
- Creatinine clearance < 40 ml/min
- Cardiac dysfunction as defined by:
- Reduced left ventricular function with an ejection fraction < 45% as measured by MUGA scan or echocardiogram (another method for measuring cardiac function is acceptable)
- Unstable angina
- Unstable cardiac arrhythmias
- Pulmonary function test with VC, FEV1 and/ or DCO < 50%
- Active, uncontrolled infection
- History of high dose (≥ 8 Gy) total body irradiation
- Pregnant or lactating females
- HIV positivity
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- By ALEA; Use goto eCRF button > select the [Patient tab] and click the [Add new patient] button. Complete all items and click the [Submit] button
- By faxing the completed registration/randomization CRF +31 (0)10 704 1028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31 (0)10 704 1560 Monday through Friday, from 09:00 to 17:00 CET
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
Site
7 results
Order by
Accrual rate
Activation date
NL-Rotterdam-EMCDANIEL
32
10 Sep 2012
NL-Utrecht-UMCUTRECHT
16
21 Aug 2013
NL-Amsterdam-VUMC
8
03 Jan 2013
NL-Amsterdam-AMC
7
22 Aug 2012
NL-Leiden-LUMC
5
01 Oct 2012
NL-Nijmegen-RADBOUDUMC
2
20 Aug 2012
NL-Maastricht-MUMC
