LYSARC HO119 NHL
Main info
- Identifier:
- HO119 MCL
- Sponsor:
- LYSARC
- Working group party:
- Lymphoma
- Age:
- >= 60
- Stage:
- 1st Line
- Echelon:
- Level D
- Included patients:
-
75(of 100)
- Active sites:
-
23(of 25)
- Title:
Efficacy of alternating immunochemotherapy consisting of RCHOP + R-HAD versus R-CHOP alone, followed by maintenance therapy consisting of additional lenalidomide with rituximab versus rituximab alone for older patients with mantle cell lymphoma
Timeline
News
HO119 status
- URL for IWRS: http://www.clininfohosting.com/specif/MCLR2_ELDERLY/
- URL for eCRF: http://study.lysarc.info/ (choose MCL-R2-Elderly)
13-SEP-2017 Updated documents:
- DSUR IV has been made available.
- IB rituximab SC v7 - addendum 1 has been made available.
- delegation log version v2.0 dd 22-may-2017 replaces the previous version.
- ABR formulier versie 07 dd 17-07-2017 replaces the previous version.
- The CCMO and METc approval documents are available for download.
27-JAN-2017
- HO119 MCL R2 Elderly protocol v5.2 dd 08-09-2016 replaces the previous version HO119 MCL R2 Elderly protocol v5.1 dd 12-10-2015.
- DSUR III, Jaarlijkse veiligheidsrapportage MCL R2 ELDERLY dd 06-07-2017 has been made available.
- IB lenalidomide V20 (and in between V19) replaces previous version IB lenalidomide V18.
- IB rituximab SC V7 replaces the previous version IB Rituximab SC V6.
- Ho119 MCL Contact sheet v3.0 27-01-2017 replaced version 2.0 04-12-2015.
- The cumulative CCMO and METc approval documents have been updated accordingly.
04-MAR-2016
- The HO119 monitoring and completion guidelines V4.0 28-OKT-2015 replaces the previous version MCL-R2 Elderly_Monitoring and completion guidelines_v2.0 20141017.
- The HO119 HOVON Subject Identification Log v2.0 16-FEB-2016 replaces the previous version 01 21-08-2013.
08-DEC-2015 - amendment 01 implemented
- HO119 MCL R2 ELderly Protocol V5.1 d.d 12-10-2015 replaces previous version V4.0 d.d. 17-03-2014
- HO119 MCLR2 Elderly-protocol signature page_v5.1 NL replaces previous version 4.0
- HO119 lenalidomide education and counseling guidance document V4.0 19Dec2014 and patient information sheet V4.0 19Dec2014 replace the previous lenalidomide education and counseling guidance document v1_31mar2015 and patient information sheet v3_mei2010
- IB lenalidomide V18 replaces previous version IB lenalidomide V17
- IB rituximab SC V6 replaces the previous version IB Rituximab SC V5
- HO119 verpleegkundige samenvatting V.26.01.2015, protocol_5.1 Study flow chart.pdf replaces the previous ho119-verpleegkundige-samenvatting-study-flow.pdf
- HO119 METC Erasmus MC approval for amendment 01 d.d. 30NOV2015 & previous approval made available
- HO119 CCMO response amendment 01 26NOV2015 + previous approval made available
- HO119 HO119 MCL Contact sheet v2.0 04DEC15 replaces the previous version Ho119 MCL Contact sheet 26JAN15
22-SEP-2015
- ho119 drug accountability forms rituximab sc and lenalidomide 05 10 15 mg.pdf made available
26-AUG-2015
- Pathology review procedure 26-AUG-2015.pdf replaces the previous version 08 jun 2015.pdf
- MRD minimal residual disease memo and manuals 26-AUG-2015.pdf replaces the previous version 08 jun 2015.pdf
- IWRS userguide for investigators, trial coordinators v1.5.pdf 13-MAY-2015 replaces previous version v1.5 13-MAY-2015 (minor update)
17-AUG-2015
- Removal from quarantine for Rituximab SC.pdf made available
- Alert form of temperature excursion_v2.0.pdf made available
- Management and administration of treatment units v1.0 01-AUG-2014.pdf made available
02-JUL-2015:
- Financial disclosure form version 1.2 replaces the previous version 1.1
- IWRS userguide for investigators, trial coordinators v1.5.pdf made available
- IWRS userguide for Pharmacists v1.3.pdf made available
- Pathology review procedure v08 jun 2015.pdf made available
28-MAY-2015:
- H119 ICF biologisch materiaal final versie 2.1 replaces the previous version 2.0
13-SEP-2017: Amendment 03 implemented
- documents approved: IB Rituximab SC v7 - addendum 1, DSUR IV
- New version of the delegation log implemented
- Changed investigators for centers UMCG-Groningen and Spaarne ziekenhuis-Hoofddorp
27-JUL-2017: Usage of biosimilars of Rituximab Mabthera IV during HO119 MCL R2 Elderly induction treatment is approved. Please refer to the information letter 'MCL-R2 Elderly_Jul 2017_use of biosimilar of Rituximab for induction tre....pdf', available for download in the 'other documents' section below.
27-JAN-2017: Amendment 02 implemented
- New versions of documents approved: protocol, IB's
- DSURIII available
08-DEC-2015: Amendment 01 implemented
- Participation of centers Zuyderland MC-Sittard-Geleen, OLVG-Amsterdam en Gemini ziekenhuis-den Helder approved
- Changed investigators for centers OLVG-Amsterdam, ADRZ-Goes and UMCG-Groningen.
- New versions of documents approved: protocol, IB's, Lenalidomide pregnancy prevention plan (among others).
29-JUL-2015: First center opened for inclusion
26-MAY-2015: Sites provided with study documents
11-MAY-2015: METc approval obtained
Flow
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Study Specific
- Objectives:
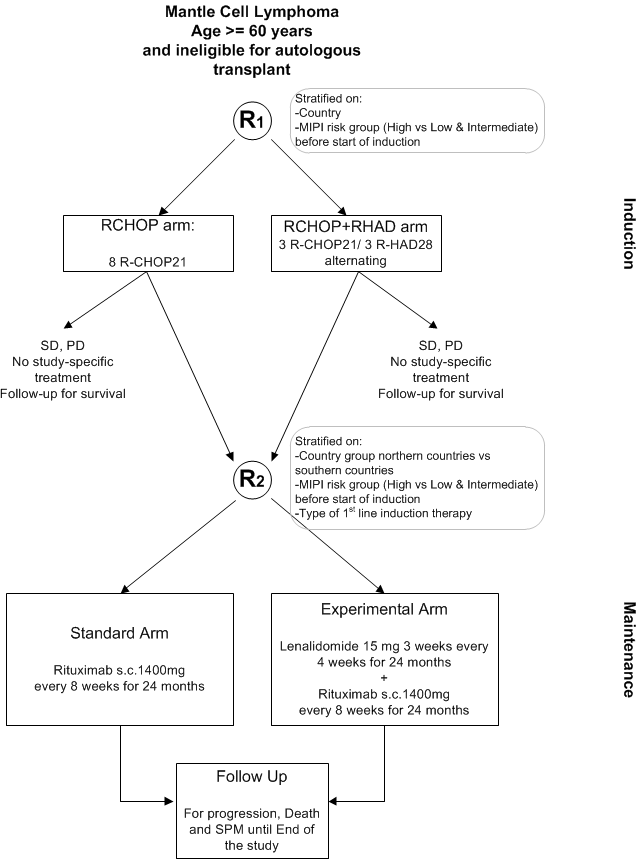
The primary objective of the trial is to evaluate whether the addition of lenalidomide to rituximab maintenance improves progression free survival (PFS) compared to standard rituximab maintenance after response to induction chemotherapy in older patients with mantle cell lymphoma not suitable for autologous stem cell transplantation.
Secondary objectives are- to compare efficacy and safety of the maintenance regimens in terms of secondary endpoints
- to evaluate whether the introduction of cytarabine into induction improves clinical outcome compared to standard R-CHOP in older patients with mantle cell lymphoma not suitable for autologous stem cell transplantation. This objective will be answered in a confirmatory way with overall survival as primary variable of interest.
- to compare efficacy and safety of the induction regimens in terms of other secondary endpoints
Eligibility
- Inclusion Criteria:
Inclusion criteria for randomization 1
Patients must satisfy all the following criteria at study entry to be randomized in the trial:- signed informed consent form
- Biopsy-proven mantle cell lymphoma according to WHO classification, including evidence of cyclin D1 overexpression or the translocation t(11;14)(q13;q32)
- ≥ 60 years of age and ineligible for autologous transplant
- Ann Arbor stage II-IV
- previously untreated (except for patients randomized directly for maintenance treatment who will receive 8 RCHOP before registration in the trial)
- ECOG performance status ≤ 2
- Male subjects must:
- agree to use a condom during sexual contact with a woman of childbearing potential, even if they have had a vasectomy, throughout lenalidomide therapy
- agree to not donate semen during lenalidomide therapy.
- All subjects must:
- have an understanding that the lenalidomide could have a potential teratogenic risk.
- agree to abstain from donating blood while taking lenalidomide therapy
- agree not to share study medication with another person.
- be counselled about pregnancy precautions and risks of foetal exposure.
Additional Inclusion criteria for randomization 2
To be randomized for maintenance, the patient must satisfy all inclusion criteria for randomization 1 and the following criteria:- CR, CRu or PR after induction treatment, determined as per Cheson 1999 criteria (see Appendix G), by investigator even for patients enrolled during the run-in phase.
- During the run-in period of 6 months starting from the date of the first randomization in the trial up to May 2014: in case of direct randomization into maintenance phase, patient must have been treated in first line by 6-8 cycles of R-CHOP.
- Exclusion Criteria:
Exclusion criteria for randomization 1
The presence of any of the following will exclude a subject from enrollment:- Female of childbearing potential (i.e. without natural menopause for at least 24 consecutive months, a hysterectomy or bilateral oophorectomy)
- Any of the following laboratory abnormalities at diagnosis, if not related to lymphoma:
- Absolute neutrophils count <1,000 /mm^3 (1.0 x 10^9/L) if not result of a bone marrow infiltration.
- Platelet count < 75,000/mm^3 (75 x 10^9/L) if not result of a bone marrow infiltration.
- Serum aspartate transaminase (AST/SGOT) or alanine transaminase (ALT/SGPT) >3.0 x upper limit of normal (ULN).
- Serum total bilirubin > 1.5 ULN (except if due to Gilbert’s syndrome)
- Calculated creatinine clearance (Cockcroft-Gault formula or MDRD) < 30 mL / min.
- Central Nervous System involvement by lymphoma
- Contraindication for medical DVT prophylaxis for patients at high risk for DVT
- Prior history of malignancies other than MCL unless the subject has been free of the disease for ≥ 5 years. Exceptions include the following:
- Basal cell carcinoma of the skin,- Squamous cell carcinoma of the skin,
- Carcinoma in situ of the cervix,
- Carcinoma in situ of the breast,
- Incidental histologic finding of prostate cancer (TNM stage of T1a or T1b).
- Any serious medical condition, laboratory abnormality, or psychiatric illness that would prevent the patient to receive the study medication as planned.
- Poor cardiac function (LVEF < 50%) on echocardiography
- Seropositivity for human immunodeficiency virus (HIV, mandatory test) at study entry
Seropositivity for hepatitis C virus (HCV, mandatory test) at study entry,
Active viral infection with hepatitis B virus (HBV, mandatory test) at study entry:- HBsAg positive
- HBsAg negative, anti-HBs positive and anti-HBc positive Patients with prior Hepatitis B must be given antiviral prophylaxis and HBV DNA monitored.
Note: Patients who are HBsAg negative, anti-HBs positive and/or anti-HBc positive but viral DNA negative are eligible.
HIV, HVC and HBV tests are mandatory- Uncontrolled illness including, but not limited to:
- Active infection requiring parenteral antibiotics.
- Uncontrolled diabetes mellitus
- Chronic symptomatic congestive heart failure (Class NYHA III or IV).
- Unstable angina pectoris, angioplasty, stenting, or myocardial infarction within 6 months
- Clinically significant cardiac arrhythmia that is symptomatic or requires treatment, or asymptomatic sustained ventricular tachycardia.
- Prior ≥ Grade 3 allergic hypersensitivity to thalidomide.
- Prior ≥ Grade 3 rash or any desquamating (blistering) rash while taking thalidomide.
- Known anti-murine antibody (HAMA) reactivity or known hypersensitivity to murine antibodies.
- Subjects with ≥ Grade 2 neuropathy.
- Prior use of lenalidomide
- Participation in another clinical trial within three weeks before randomization in this study
Additional exclusion criteria for randomization 2
To be randomized for maintenance, the patient must satisfy all exclusion criteria for
randomization 1 and the following criteria:- The presence of any exclusion criteria of randomization 1 or of the following criteria will exclude a subject from enrollment in the maintenance phase:
- SD or PD after induction treatment determined as per Cheson 1999 criteria (see Appendix G) by investigator.
- Patient treated by induction immuno-chemotherapy other than 6-8 cycle of R-CHOP21 or 2-3 cycles of R-CHOP21 / 2-3 cycles of R-HAD28 (alternating)
- Patients with serious underlying medical conditions, which could impair the ability to receive maintenance treatment
- Calculated creatinine clearance (Cockcroft-Gault formula or MDRD) of < 30 mL / min at screening for maintenance.
- ANC is < 1,000 cells/mm³ (1.0 X 10^9/L) at screening for maintenance;
- Platelet count is < 50,000 cells/mm³ (50 X 10^9/L) at screening for maintenance.
Registration Details
Registration for participating in the study is done according two pathways:
- Previously untreated patients will be registered and randomized for induction before the first cycle of induction treatment. Patients who reached CR, CRu or PR after induction treatment, will have a second randomization for the maintenance treatment.
- During a run-in period of 6 months starting from the date of the first patient randomized in the trial, it was possible to randomize patients directly for maintenance treatment after being treated in first line by 8 cycles of R-CHOP and after having reached CR, CRu or PR (determined as per Cheson 1999 criteria (see Appendix G). This run-in period was closed in May 2014. In these patients, the data collection regarding the R-CHOP part will be done retrospectively.
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
