HOVON HO124 Other
Closed
Main info
- Identifier:
- HO124 WM
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- >= 18
- Stage:
- 2nd Line
- Echelon:
- Level B
- Included patients:
-
60(of 72)
- Active sites:
-
21(of 22)
- Title:
HOVON 124 WM/ECWM-R2 study: A prospective phase I/II trial of the combination of ixazomib citrate, rituximab and dexamethasone in patients with relapsed or progressive Waldenström’s macroglobulinemia. A HOVON/Greek Myeloma Study Group study.
Timeline
Scheduled
Actual
2013
01 Jan
Opportunity
2014
19 Sep
EC Approval
2014
02 Dec
Activated
2014
02 Dec
First Site
2015
12 Jan
FPI
2019
02 Jan
ClosedForInclusionActualStart
2024
31 Aug
Endpoint Analysis
2025
30 Apr
Archived
2025
26 Nov
CloseoutInProgressLastPtOutActualStart
News
Study results are published at: https://pubmed.ncbi.nlm.nih.gov/34388022/
Flow
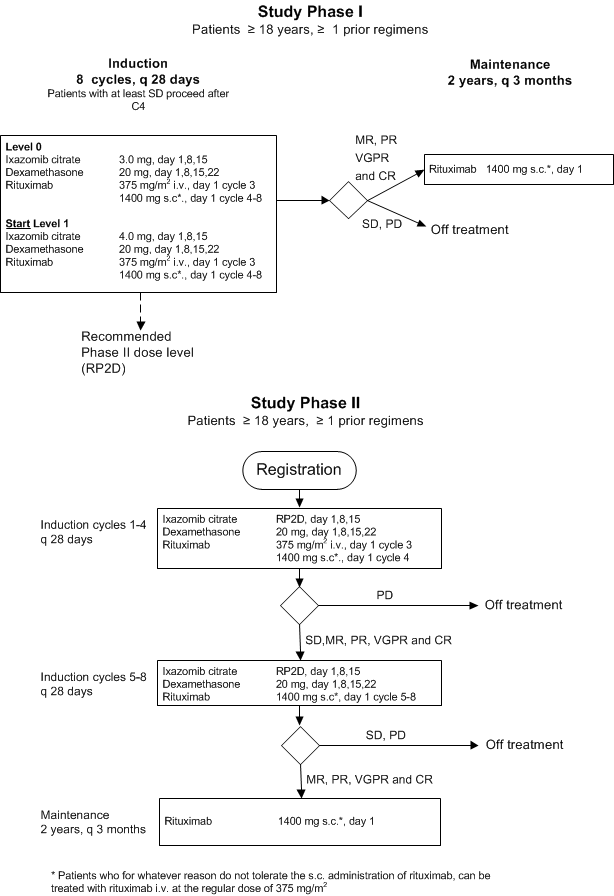
Details
- Phase:
- Prospective Phase I/II study
- Monitoring Type:
- Not any more
- Objectives:
Phase I part:
- Establish maximum tolerated dose for the combination of ixazomib citrate and dexamethasone
Phase II part:
- Assess the efficacy of oral ixazomib citrate in combination with rituximab and dexamethasone
Eligibility
- Inclusion Criteria:
- A diagnosis of WM (lymphoplasmacytoid lymphoma in the bone marrow with an IgM M-protein)
- Age ≥ 18 years
- WHO 0-2
- Presence of an IgM M-protein in the serum (as demonstrated by SPEP and IF)
- Measurable disease (IgM M-protein > 10 g/l (1 g/dl or, in case the M-protein is present but unquantifiable, total serum IgM level > 10 g/l (1 g/dl))
- Symptomatic disease
- ≥ 1 prior lines of treatment
- Patients showing progressive disease under treatment with chemotherapy only (e.g. chlorambucil, CVP, fludarabine or fludarabine/cyclophosphamide) are allowed on study
- Platelets > 75x109/l. Platelet transfusions to help patients meet eligibility criteria are not allowed within 3 days before study enrollment.
- ANC >1.0 x109/l
- Negative pregnancy test at study entry for women of childbearing potential
- A female patient is either post-menopausal for at least 1 year before the screening visit, or surgically sterile, or, if of childbearing potential, agrees to practice 2 effective methods of contraception at the same time, from the time of signing the informed consent until 12 months after the last dose of rituximab, or agrees to completely abstain from heterosexual intercourse. Periodic abstinence (e.g. calendar, ovulation, symptothermal, post-ovulation methods) and withdrawal are not acceptable methods of contraception)
- Male patients, even if surgically sterilized, (i.e., status post vasectomy) must agree to practice effective barrier contraception during the entire study period and through 90 days after the last dose of ixazomib and/or rituximab, or agree to completely abstain from heterosexual intercourse. Periodic abstinence (e.g. calendar, ovulation, symptothermal, post-ovulation methods) and withdrawal are not acceptable methods of contraception)
- Written informed consent
- Prior plasmapheresis in case of symptomatic hyperviscosity is allowed
- Exclusion Criteria:
- Bortezomib refractory (no PR/CR after treatment with bortezomib, and/or progression within 6 months of treatment with bortezomib)
- Rituximab refractory (progressive disease during treatment or within 6 months after last administration of rituximab)
- WHO performance status >2
- Amyloidosis
- Peripheral neuropathy, grade 3 or higher
- Creatinine clearance <30 ml/min
- Known HIV seropositivity
- Patients with active hepatitis B or C infection
- Known intolerance of rituximab and/or boron
- Bing Neel syndrome or other severe neurological disorders
- Severe cardiac dysfunction (NYHA classification III-IV; see appendix….)
- Severe pulmonary dysfunction (CTCAE grade III-IV, see appendix …)
- Significant hepatic dysfunction (defined as total bilirubin >1.5 ULN (unless caused by Gilbert syndrome) and/or transaminases ≥3 times upper limit of normal, unless caused by WM
- Active, uncontrolled infections
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, hypertension, cancer)
- A history of other malignancies, unless the malignancy has been in remission for at least 5 years (with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma)
- Pregnancy or breastfeeding
- Current participation in another clinical trial
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- By ALEA; Use goto eCRF button > select the [Patient tab] and click the [Add new patient] button. Complete all items and click the [Submit] button
- By faxing the completed registration/randomization CRF +31 (0)10 704 1028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31 (0)10 704 1560 Monday through Friday, from 09:00 to 17:00 CET
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
Site
21 results
Order by
Accrual rate
Activation date
GR-Athens-ALEXANDRA
14
23 Feb 2016
NL-Amsterdam-VUMC
7
08 Jun 2015
BE-Roeselare-AZDELTA
5
01 Mar 2016
NL-Eindhoven-MAXIMAMC
5
01 Jun 2015
NL-Rotterdam-EMCDANIEL
4
28 May 2015
NL-Utrecht-UMCUTRECHT
4
11 Jan 2016
BE-Gent-UZGENT
3
14 Mar 2016
NL-Den Haag-HAGA
3
20 Jul 2015
NL-Groningen-UMCG
2
30 May 2016
NL-Amsterdam-AMC
2
02 Dec 2014
NL-Hilversum-TERGOOI
2
13 Feb 2017
NL-Leiden-LUMC
2
23 Jul 2015
BE-Antwerpen-ZNASTUIVENBERG
2
02 Mar 2016
NL-Capelle a/d IJssel-YSL
1
15 Dec 2016
NL-Leeuwarden-MCL
1
30 Mar 2017
NL-Rotterdam-ERASMUSMC
1
29 Oct 2014
NL-Nieuwegein-ANTONIUS
1
19 May 2015
NL-Maastricht-MUMC
1
26 Jan 2016
BE-Leuven-UZLEUVEN
01 Sep 2016
NL-Zwolle-ISALA
20 Jul 2015
BE-Turnhout-AZSTELISABETH
27 Dec 2016
