HOVON HO127 NHL
Main info
- Identifier:
- HO127 BL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- 18-75
- Stage:
- 1st Line
- Echelon:
- Level C-HIC&C-SCT
- Included patients:
-
89(of 260)
- Active sites:
-
23(of 46)
- Title:
Phase III study comparing R-CODOX-M/R-IVAC versus dose-adjusted EPOCH-R (DAEPOCH-R) for patients with newly diagnosed high risk Burkitt lymphoma
Timeline
News
15-NOV-2021: the study is closed for inclusion, from November 15th 2021.
Patients who have started protocol treatment can continue their treatment according to the protocol.
The follow up of the study is 5 years.
12FEB2021:There is an updated SAE_v04 form available on the HOVON-website.
Flow
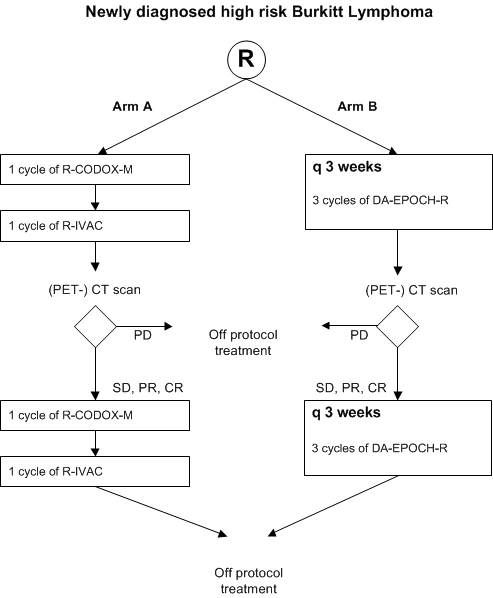
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Site Evaluation Visit
- Objectives:
Primary objective
- To confirm in a multi-centre phase III setting an improvement in PFS to 85% at 2 years of DAEPOCH-R in patients with newly diagnosed high risk Burkitt lymphoma as compared to an expected PFS of 70% at 2 years for the control arm R-CODOX-M/R-IVAC
Secondary objectives
- To evaluate Overall Response Rate (ORR) end-of- treatment, Event Free Survival (EFS) and Overall Survival (OS) at 2 years
- To evaluate both regimens with respect to CTCAE grade ≥3 toxicity
- To evaluate both regimens with respect to hospitalisation days
Eligibility
- Inclusion Criteria:
- First diagnosis of high risk Burkitt lymphoma (sporadic and HIV associated), histologically confirmed according to the WHO classification 2008. Upon its availability the WHO 2016 classification should be used, to replace the WHO 2008 classification
- High risk disease; i.e. any of following:
- elevated LDH
- WHO performance status ≥ 2 (appendix C)
- Ann Arbor stage III or IV (Appendix A)
- tumour mass ≥ 10 cm
- Age 18-75 years inclusive
- WHO performance status (PS) 0-3, WHO PS 4 only if disease related (Appendix C)
- Written informed consent
- Exclusion Criteria:
- All histopathological diagnoses other than Burkitt lymphoma according to the WHO classification 2008, irrespective of the presence of a MYC rearrangement; Upon its availability the WHO 2016 classification should be used, to replace the WHO 2008 classification
- Patients with endemic Burkitt lymphoma
- Patients with low risk Burkitt lymphoma (i.e. all of following: normal LDH, WHO performance status 0 or 1 (appendix C), Ann Arbor stage I or II (Appendix A), no tumour mass ≥ 10 cm)
- Patients with CNS localisation of Burkitt lymphoma
- Prior treatment other than local radiation (max. 10 Gy) or short course (max 7 days) of steroids ≤ 1 mg/kg or ≤100mg predniso(lo)ne (whichever is greater; or equivalent corticosteroid) for acute symptoms; or 1 cycle of R-CHOP
- Creatinine clearance < 50 ml/min unless lymphoma related
- Inadequate hepatic function: bilirubin > 2.5 * ULN (total) except patients with Gilbert’s syndrome as defined by > 80% unconjugated
- Inadequate haematological function ANC < 1x10^9/l and platelets < 75x10^9 /l unless lymphoma related
- Severe pulmonary dysfunction (CTCAE grade 3-4, see appendix D)
- Severe neurological or psychiatric disease
- Active symptomatic ischemic heart disease, myocardial infarction, or congestive heart failure within the past year. If an ultrasound or MUGA scan is obtained the LVEF should exceed 45%
- All men and all women of child-bearing potential not willing or able to use an acceptable method of birth control for the duration of the study and one year beyond treatment completion
- Female subject pregnant or breast-feeding
- History of a prior invasive malignancy in the past 5 years with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma
- Serious concomitant medical illnesses that would jeopardise the patient's ability to receive the regimen with reasonable safety, including active hepatitis B (HBV, see also paragraph 9.3) or hepatitis C (HCV) infection
- Current participation in another clinical trial if interfering with HO127
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule.
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- By ALEA; Use goto eCRF button > select the [Patient tab] and click the [Add new patient] button. Complete all items and click the [Submit] button
- By faxing the completed registration/randomization CRF +31 (0)10 704 1028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31 (0)10 704 1560 Monday through Friday, from 09:00 to 17:00 CET
The interim analysis is planned when treatment data of the first 50 registered and eligible patients per treatment arm are available and have been evaluated.
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
