HOVON HO129 PCL
Main info
- Identifier:
- HO129 PCL
- Sponsor:
- HOVON
- Working group party:
- Myeloma
- Age:
- >= 18
- Stage:
- 1st Line
- Echelon:
- Level A
- Included patients:
-
61(of 61)
- Active sites:
-
25(of 28)
- Title:
Carfilzomib and lenalidomide-based treatment for younger and elderly newly diagnosed primary plasma cell leukemia patients.
Timeline
News
13-AUG-2025: All sites are officially closed for the study now.
20-Jan-2024: Last patient last visit took place on 24-Dec-2024 and the study is completed.
Flow
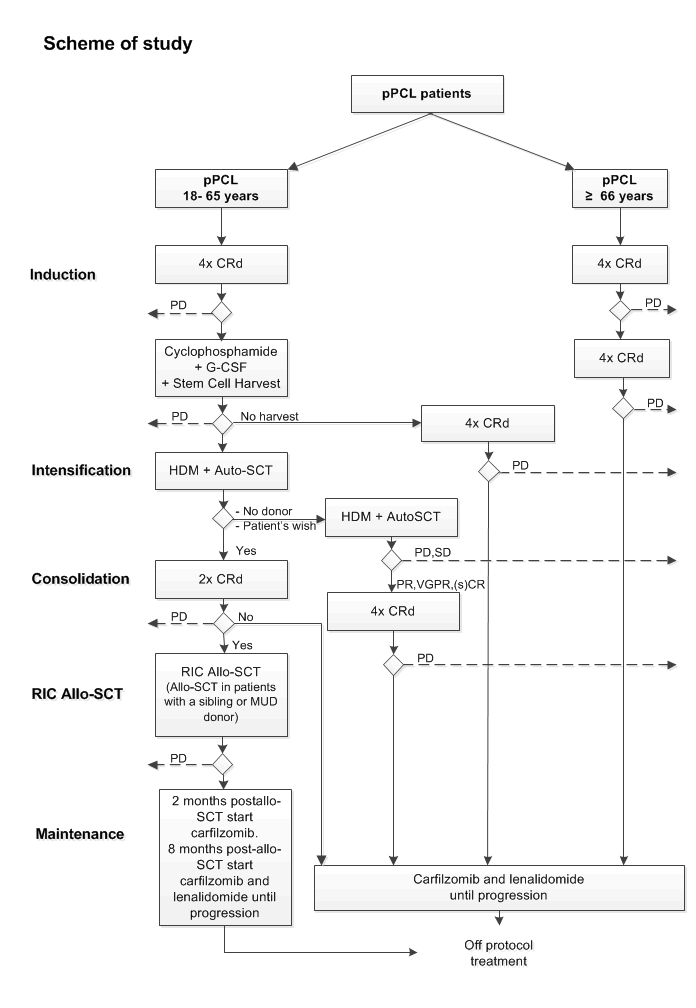
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
Primary Objective
- To evaluate progression-free survival in adult pPCL patients by incorporation of carfilzomib and lenalidomide in induction, consolidation, and maintenance therapy
Secondary objectives
- To assess overall response rate and (s)CR + VGPR ((stringent) complete and very good partial response) rate after induction therapy, after HDM, after CRd consolidation, after RIC allo-SCT or a second HDM, and during maintenance
- To evaluate overall survival
- To assess safety and toxicity
- To assess the prognostic value of risk factors at diagnosis, including β2-microglobulin, LDH, FISH abnormalities del1p, ampli 1q, t(4;14), t(14;16), t(11;14), ampli 9, del13q/13-, del17p as analyzed in purified bone marrow plasma cells with respect to progression-free survival
- To analyze the prognostic value of myeloma gene expression profiles
- To analyze the prognostic value of minimal residual disease negativity
- To assess the prognostic value of mutations as determined by sequencing
- To establish the frequency of second primary malignancies
Eligibility
- Inclusion Criteria:
- Patients with diagnosis of symptomatic pPCL (see appendix A)
- Measurable disease as defined by the presence of M-protein in serum or urine (serum Mprotein > 5 g/l or urine M-protein > 200 mg/24 hours or abnormal FLC ratio with involved free light chain (FLC) > 100 mg/l) or proven plasmacytoma by biopsy)
- Age ≥18 years
- WHO-performance status 0-3 (but WHO=3 is allowed only when caused by pPCL and not by co-morbid conditions)
- Written informed consent
- Patient capable of giving informed consent (patient is legally, physically and mentally capable of giving consent)
- All men and women of childbearing potential should use highly effective contraception during the study. Men should be offered sperm banking before starting treatment (if applicable).
- Negative pregnancy test at entry (if applicable)
- Patient is willing and able to adhere to the requirements of the lenalidomide Pregnancy Prevention Program (PPP) throughout study treatment
- Exclusion Criteria:
- *Any current CNS involvement with disease refractory to intrathecal chemotherapy
- Female patients who are pregnant or breast feeding
- HIV positive patients
- Active malignancy other than pPCL requiring treatment, or a malignancy that has been treated with chemotherapy currently affecting bone marrow capacity
- Patients with active, uncontrolled infections
- Severe neurological or psychiatric disease
- Severe cardiac dysfunction (NYHA classification II-IV, see appendix E)
- Myocardial infarction within 6 months
- unstable angina
- cardiac arrhythmias which are not controlled by conventional treatment (including medications and cardiac devices)
- Severe pulmonary dysfunction
- Significant hepatic dysfunction (serum bilirubin or transaminases ≥ 3.0 times normal level), unless related to pPCL
- Patients with GFR < 15 ml/min
- Known history of allergy to Capsidol (a cyclodextrin derivative used to solubilize carfilzomib)
- Hypersensitivity to the active substances (carfilzomib, lenalidomide, and dexamethasone) or to any of the excipients of these drug products
- Previous chemotherapy or radiotherapy except local radiotherapy in case of local myeloma progression or corticosteroids maximum 7 days for symptom control or stabilization (this includes dexamethasone 40 mg daily) or intrathecal chemotherapy in case of CNS involvement
- Systemic AL amyloidosis
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule
Registration Details
The patient should be registered immediately after diagnosis and before the start of protocol treatment.
Patients will be registered at EMN Research Italy by web http://www.emntg.org. Investigators who do not have an account yet should register at this website to obtain an account after consultation of HDC.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of responsible investigator
- Age on inclusion
- Date of informed consent
- Date of sample shipment (optional)
- Date of diagnosis of pPCL
- Criteria for measurable disease
- Serum β2-microglobulin
- Serum albumin
- Eligibility criteria
For each of the two age groups, one interim analysis is planned, primarily to describe adverse events and response observed during induction therapy with CRd. This will be done when complete data of the first 15 registered patients (elderly and younger patients separately) regarding CRd cycles 1-4 are available.
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
