HOVON HO130 NHL
Main info
- Identifier:
- HO130 NHL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- >= 18
- Stage:
- 1st Line
- Echelon:
- Level D
- Included patients:
-
85(of 85)
- Active sites:
-
23(of 23)
- Title:
A phase II study evaluating the effect of the addition of lenalidomide to R-CHOP for patients with newly diagnosed MYC positive DLBCL and BCL-U.
Timeline
News
- 23-FEB-2018 the study has completed patient inclusion: The HOVON 130 NHL trial has been closed for further patient recruitment. The target number of 85 registered patients has been reached.
Please find below the official notification letter for closure of the study.
Please inform your pharmacy so that they can accommodate their IMP lenalidomide stock to the fact that no additional patients are to be expected anymore.
The patients that have been included should be treated and followed according to protocol. The required follow up period for all patients is 5 years from registration.
The PI dr. M. Chamuleau and the HOVON 130 study team want to thank you wholeheartedly for your efforts and help during the recruitment phase of the trial.
Our next aim is to complete patient study data collection. Our ambition is timely availability of the patient study data. It would be very helpful if the eCRF up-to-date status could be maintained and the PET/CT scans are provided for central review upon their availability.
- link to the eCRF: https://aleaclinical.com/hovon/dm/ (log in required)
Updated documents:
25-jul-2017: For The Netherlands only 18-dec-2017: Belgium approval
- HO130 NHL protocol v6 18-may-2017 final _signed.pdf replaces the previous version HO130 NHL protocol v5 19-06-2016 final_signed is available for the Dutch participating centers
- ho130 ICF v6 18may2017 final voor sites.doc replaces the previous version HO130 ICF v5 12jun2015 voor site, for the Dutch participating centers
- HO130 NHL Positive decision letter METC VUmc for amendment 06 18-jul-2017 is available for the Dutch participating centers (added to cumulative document)
- HO130 CCMO no objection statement amendment 06 11-jul-2017 is available for the Dutch participating centers (added to cumulative document)
- HO130 NHL CRF v9 24-jul-2017 replaces the previous version for the Dutch participating centers
- HO130 Instructions for e-CRF completion version 17-jul-2017 replaces the previous version for the Dutch participating centers.
18-AUG-2016
*HO130 NHL protocol v5 19-06-2016 final_signed replaces the previous version HO130 NHL protocol v4 21-05-2016 (for all participating countries)- HO130 NHL Positive decision letter METC VUmc for amendment 04 17-AUG-2016 is available for the Dutch participating centers. (attached to cumulative document)
- HO130 NHL CCMO no objection statement amendment 04 12-AUG-2016 is available for the Dutch participating centers. (attached to cumulative document)
- HO130 NHL ABR-formulier versie 07 30-JUN-2016 replaces the previous version ABR versie 06 dd 05-APR-2016.pdf
23-may-2016
- HO130 METc positief oordeel site amendement 3 NL50964.029.14 17-MAY-2016.pdf is available (attached to cumulative document)
- HO130 CA goedkeuring_site amendement 3 20-MAY-2016.pdf is available. (attached to cumulative document)
- HO130 ABR versie 06 dd 05-APR-2016.pdf is available.
13-may-2016
- HO130 Instructions for e-CRF completion version 19-APR-2016.pdf replaces the previous version HO130 Instructions for e-CRF completion version 29-JAN-2016.pdf
- HOVON 130 NHL-CRF final v7 11-may-2016.pdf replaces HOVON 130 NHL CRF final v6 16-FEB-2016.pdf
18-feb-2016
- HO130 Instructions for e-CRF completion version 29-JAN-2016.pdf made available
- HO130 HOVON 130 NHL CRF final v6 16-FEB-2016.pdf replaces previous version HOVON 130 NHL CRF final v5 11DEC2015.pdf
- HO130 NHL-registration-form v6 16-FEB-2016.pdf replaces previous version HO130 NHL-registration-form v5 11DEC2015
22-dec-2015
- HOVON 130 NHL CRF final v5 11DEC2015.pdf replaces previous version HOVON 130 NHL CRF final v4 01APR2015.pdf
- HO130 NHL-registration-form v5 11DEC2015 replaces previous version HOVON 130 NHL Registration form 01APR2015
17-nov-2015
- New IDOS manual HO130_IDOS_Training_Shipment to Participant Sites_V5_07-okt-2015 replaces previous version 3.1
Update 18-DEC-2017: Amendment approved for The Netherlands on 25-JUL-2017 was also approved for Belgium.
Update 25-JUL-2017: Amendment 06 containing an updated protocol, ICF, IB Lenalidomide v21 and approval for usage for rituximab IV biosimilars was approved and communicated to the participating centers in The Netherlands only. Amendment for Belgium is pending.
Update 14-NOV-2016: Amendement 02 BE containing 'IB lenalidomide V19', 'IB lenalidomide V20' and 'study protocol v5 19-06-2016 final' been CA/EC approved for participating centers in Belgium
Update 18-AUG-2016: Amendment 04 containing 'IB lenalidomide V19' and 'study protocol v5 19-06-2016 final' been CA/EC approved for participating centers in The Netherlands.
Update 23-MAY-2016: Site amendment 03 has been EC/CA approved. This amendment concerns only the participating centers in The Netherlands.
Update 18-feb-2016: Activated centers have been informed about the availability of the ALEA eCRF database for the HOVON 130 NHL study
Update 17-nov-2015: Various centers have opened for inclusion
Update 14-jul-2015: De studie is geopend. Deelnemende sites zijn aan het opstarten.
Update 14-apr-2015: De studie is op dit moment alleen open in VUMC.
Er wordt een amendment voorbereid en ingediend, alvorens de overige sites kunnen starten.
Flow
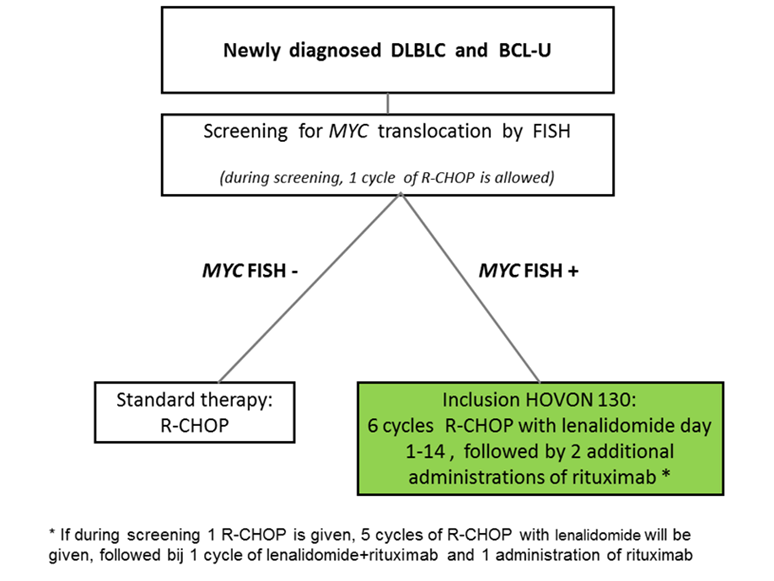
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
Primary objective
To evaluate the efficacy of the combination of lenalidomide and R-CHOP in MYC+ DLBCL patients in terms of CR rate by end-of-treatment F-FDG PET-CT scan and BM.For DH -DLBCL patients the complete remission rate on R-CHOP was reported by Green at 64%in 2012. Later publications showed CR rates of 35%, 40% and 50%. For all DLBCL patients the CR/Cru rate after R-CHOP will be 58-63%. In this protocol we aim to improve the CR rate by end-of-treatment as assessed by F-FDG PET-CT from 45% to 60%.
Secondary objectives
To evaluate the efficacy of the addition of lenalidomide to R-CHOP for MYC+ DLBCL patients in terms of EFS, DFS and OS.To evaluate the value of mid-treatment F-FDG PET-CT scanning in predicting end-of-treatment F-FDG PET-CT result.
Eligibility
- Inclusion Criteria:
- DLBCL or BCL-U, histologically confirmed according to the WHO classification 2008 with a MYC rearrangement as determined by FISH comprising:
- single hit (SH MYC+ lymphoma, not fulfilling the criteria for Burkitt Lymphoma ) or
- double hit lymphoma (DH) MYC+/BCL2+ or MYC+/BCL6+ or
- triple hit lymphoma (TH) MYC+/BCL2+/BCL6+
- Age ≥ 18 year
- No prior treatment except
- local radiation or short course (max 7 days) steroids (max 100 mg/day)
- 1 course of R-CHOP in case MYC positivity became evident during first cycle of treatment
- WHO performance status (PS) 0-3, status 4 only if disease related (see appendix C)
- Ann Arbor stage II-IV
- Measurable disease: on contrast-enhanced CT scan at least 1 lesions/node with a long axis of >1.5 cm and at least one positive lesion on 18F-FDG PET scan.
- Negative pregnancy test at study entry
- Patient is willing and able to adhere to the requirements of the lenalidomide Pregnancy Prevention Risk Management Program
- Written informed consent
- Patient is capable of giving informed consent.
- DLBCL or BCL-U, histologically confirmed according to the WHO classification 2008 with a MYC rearrangement as determined by FISH comprising:
- Exclusion Criteria:
- All histopathological diagnoses other than DLBCL not otherwise specified or BCL-U according to the WHO classification 2008 (like Burkitt lymphoma, DLBCL associated with chronic inflammation), irrespective of the presence of MYC rearrangement
- Known history of indolent lymphoma, or presence of indolent lymphoma in the diagnostic lymph node. If during screening localization of an indolent lymphoma in the bone marrow biopsy is diagnosed, the patient is eligible.
- Absence of contrast-enhanced CT scan (neck, thorax, abdomen, pelvis) and 18F-FDG PET scan performed before first cycle of R-CHOP
- Inadequate renal function or creatinine clearance < 30 mL/min (after rehydration). Creatinine clearance may be calculated by Cockcroft –Gault formula: CrCl = (140 - age [in years]) x weight [kg] (x 0.85 for females)/ (0.815 x serum creatinine [μmol/L])
- Inadequate hepatic function: bilirubin > 3 times ULN (total) except patients with Gilbert's syndrome as defined by > 80% unconjugated bilirubin
- Inadequate hematological function: ANC < 1.0x10^9/L or platelets < 75x10^9 /L unless lymphoma related
- CNS localization of the lymphoma. Liquor analysis before start of treatment is only necessary in case of suspicion
- Female subject pregnant or breast-feeding
- History of active malignancy during the past 5 years with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma
- Active symptomatic ischemic heart disease, myocardial infarction, or congestive heart failure within the past year. If echo or MUGA is obtained the LVEF should exceed 40%
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, infection, hypertension, cancer, etc.) that would jeopardize the patient's ability to receive the regimen with reasonable safety
- HIV positivity
- Active Hepatitis B or C infection as defined by positive serology and transaminitis. Non-active Hepatitis B carriers may be included if protected with lamivudine (see 9.4)
- Severe pulmonary dysfunction (CTCAE grade III-IV, see appendix D)
- Severe neurological or psychiatric disease
- Current participation in another clinical trial interfering with this trial
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule
- Claustrophobia to the extent that PET-CT is impossible.
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- Trial Online Process (TOP, https://www.hdc.hovon.nl/top). A logon to TOP can be requested at the HOVON Data Center for participants.
- By faxing the completed registration/randomization CRF +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31.10.7041560 Monday through Friday, from 09:00 to 17:00 CET.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Local patient code (optional)
- Sex
- Age at registration
- Date written informed consent
- Date start previous R-CHOP treatment (if first R-CHOP cycle given before registration)
- Specific items patient gives consent for (see ICF)
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
