IFM HO131 MM
Archived
Main info
- Identifier:
- HO131 MM / IFM 2015-01 (Cassiopeia)
- Sponsor:
- IFM
- Working group party:
- Myeloma
- Age:
- 18-65
- Stage:
- 1st Line
- Echelon:
- Limited Site Selection
- Included patients:
-
176(of 300)
- Active sites:
-
35(of 29)
- Title:
Study of Daratumumab (JNJ-54767414 (HuMax® CD38) in Combination with Bortezomib (VELCADE), Thalidomide, and Dexamethasone (VTD) in the First Line Treatment of Transplant Eligible Subjects with Newly Diagnosed Multiple Myeloma.
Timeline
Scheduled
Actual
2015
01 Oct
Opportunity
2015
15 Oct
Activated
2015
15 Oct
EC Approval
2015
15 Oct
First Site
2015
14 Dec
FPI
2017
01 Aug
ClosedForInclusionActualStart
2024
26 Mar
Endpoint Analysis
2025
02 Jun
Archived
Flow
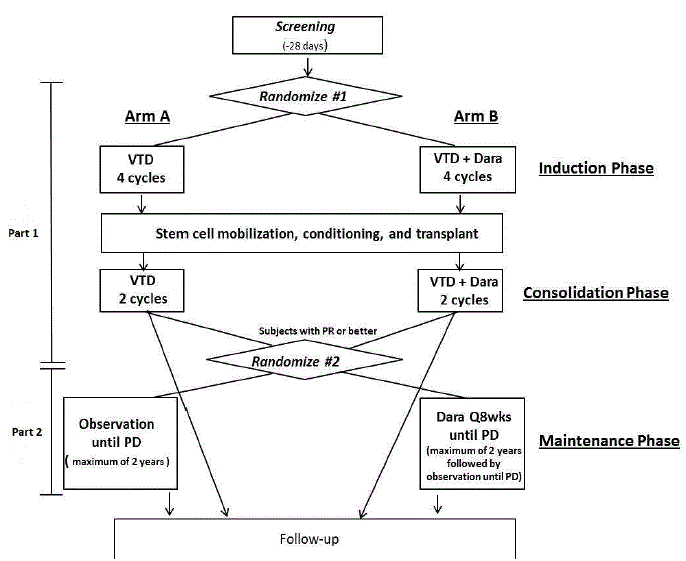
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Study Specific
- Objectives:
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
Site
35 results
Order by
Accrual rate
Activation date
NL-Rotterdam-ERASMUSMC
24
NL-Amsterdam-VUMC
15
BE-Leuven-UZLEUVEN
13
NL-Heerlen-ATRIUMMC
12
NL-Alkmaar-NWZ
8
NL-Amersfoort-MEANDERMC
7
NL-Dordrecht-ASZ
7
NL-Utrecht-UMCUTRECHT
7
NL-Groningen-UMCG
6
BE-Antwerpen-ZNASTUIVENBERG
6
BE-Haine-Saint-Paul-JOLIMONT
6
07 Dec 2016
NL-Nieuwegein-ANTONIUS
6
NL-Tilburg-ETZ
5
BE-Brugge-AZBRUGGE
5
19 Jan 2016
NL-Rotterdam-MAASSTADZIEKENHUIS
4
NL-Enschede-MST
4
NL-Deventer-DZ
4
BE-Turnhout-AZSTELISABETH
3
NL-Ede-ZGV
3
NL-Arnhem-RIJNSTATE
3
NL-Leiden-LUMC
3
NL-Den Haag-HAGA
3
NL-Eindhoven-MAXIMAMC
3
NL-Maastricht-MUMC
3
NL-Leeuwarden-MCL
3
NL-Nijmegen-RADBOUDUMC
2
NL-Amsterdam-AMC
2
NL-Hilversum-TERGOOI
2
NL-Amsterdam-OLVG
2
NL-Breda-AMPHIA
1
BE-Roeselare-AZDELTA
1
NL-Delft-RDGG
1
NL-Zwolle-ISALA
1
NL-Hoofddorp-SPAARNEGASTHUIS
1
NL-Nijmegen-CWZ
