HOVON HO139 CLL
Main info
- Identifier:
- HO139 CLL
- Sponsor:
- HOVON
- Working group party:
- CLL
- Age:
- >= 18
- Stage:
- 1st Line
- Echelon:
- Level D
- Included patients:
-
70(of 70)
- Active sites:
-
25(of 25)
- Title:
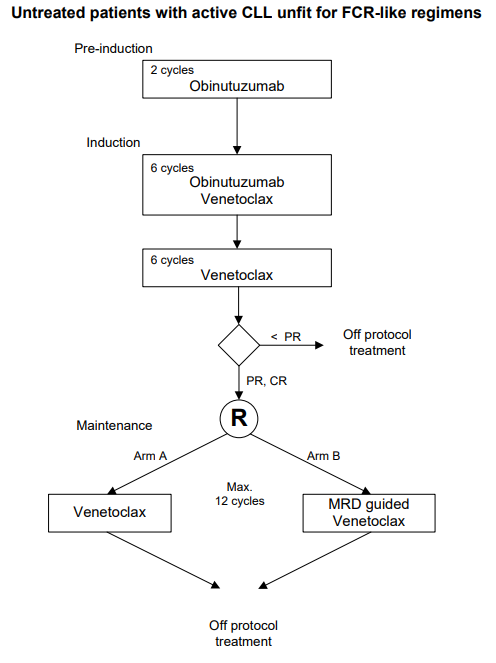
A prospective, open-label, multicenter randomized phase-II trial to evaluate the efficacy and safety of a sequential regimen of Gazyvaro (obinutuzumab) followed by obinutuzumab and venetoclax, followed by either standard venetoclax maintenance or MRD guided venetoclax maintenance in first-line patients with CLL and unfit for FCR-like regimens. (GIVE trial).
Timeline
News
13NOV2018 - updated documents:
- HOVON139 Lab manual vs. 10 dd 20181011
- HOVON139 Central-lab shipment form PB-BM dd 20181011
- Due to a change in address of the AMC Central Lab new versions of the HOVON139 lab manual and PB-BM shipment forms available
08OCT2018
- HOVON139 VERSION 5.1 PROTOCOL ACTIVE
- Local Investigators must send in a singed Protocol Signature page as soon as possible.
25JUN2018 - updated documents:
- Due to change in HOVON Data Center address. Please send all Assessments to the address below:
- HOVON Data Center (Ee2155)
- Erasmus MC Cancer Institute
- P.O.Box. 3000 CA Rotterdam
01MAY2018 - updated documents:
- HO139_Central lab shipment form LN biopsy T1 20180410
- HO139_Central lab shipment form PB-BM 20180410
- HO139_Lab manual_ v8 20180410
20APR2018
HOVON139 patient screening list for the last 10 patients
Patient registration in TOP is exclusively done via the HOVON Data Center (HDC). If you have a patient in screening for the HO139 study, please inform HDC by email
Please provide in this email:
1. the year of birth
2. the planned date when expected to register the patient in the HO139
3. the planned start date for the first HO139 cycle.
Your patient will be added to the “HOVON139 patient screening list”. Only patients listed on the “HO139 patient screening list” who are eligible will actually be registered.
For registering a patient you must complete the HOVON139 MM registration form and fax it to the HOVON Data Center
Please inform us as soon as possible if the (your) patient listed on the screening list turns out not to be eligible during screening for the HO139.
19JUN2017 - updated documents:
- AM01 has been approved by EC & CA!
All sites have been informed by e-mail and local documentation is currently being gathered
28OCT2016
Study has been approved by EC & CA!
All sites have been informed by e-mail and local documentation is currently being gathered
Flow
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
The primary objective
To separately study the efficacy, defined as MRD negative bone marrow and no progression according to the IWCLL criteria, of the two arms of the study of either venetoclax maintenance or MRD-guided venetoclax maintenance after sequential regimens of
obinutuzumab (pre-induction) followed by 6 cycles obinutuzumab with venetoclax and 6 cycles of venetoclax (induction) in first-line patients with CLL and unfit for FCR-like regimens.Secondary objectives:
- To determine efficacy as assessed by additional outcome measures, including overall response, PFS, event free survival (EFS), OS
- To determine the impact of the study treatment on quality of life and geriatric scores (including a biological senescence marker of skin biopsy)
- Toxicity of venetoclax after pre-induction, especially tumorlysis and neutropenia
- To identify predictive factors for response and resistance mechanisms via:
- Next-generation sequencing (NGS) at baseline and at progression
- Flow-based subset analysis on expression levels of Bcl-2 proteins at baseline, during therapy and at progression
- Analyses of malignant and non-malignant immune cells in PB and in LN at baseline and during treatment
Eligibility
- Inclusion Criteria:
- Diagnosis of symptomatic CLL (according to IWCLL guidelines, including minimal required markers (CD5/CD19/CD23 triple positive with light chain restriction))
- Patients without prior treatment for CLL (Corticoid treatment administered due to necessary immediate intervention is allowed; within the last 10 days before start of study treatment only dose equivalents of maximum 20 mg prednisolone are permitted);
- Patients aged ≥ 18 years, not fit for FCR-like regimens, according to the treating physician;
- Able to adhere to the study visit schedule and other protocol requirements;
- WHO performance status of ≤ 2 (see appendix C);
- Laboratory test results within these ranges:
- absolute neutrophil count ≥ 1.0 x 10^9/l and platelet count ≥ 50 x 10^9/l,
unless due to bone marrow infiltration,
- creatinine clearance ≥ 45 ml/min (using 24-hour creatinine clearance or modified Cockcroft−Gault equation (see appendix E)
- total bilirubin ≤ 1,5 x ULN unless considered due to Gilbert’s syndrome,
- transaminases ≤ 3 x ULN;
- Negative serum or urine pregnancy test within 28 days prior to registration (all females of childbearing potential);
- Written informed consent
- Patient is capable of giving informed consent
- Exclusion Criteria:
- Current inclusion in other clinical trials
- Intolerance of exogenous protein administration;
- History of severe allergic or anaphylactic reactions to humanized or murine monoclonal antibodies. Known sensitivity or allergy to murine products.
- Positive hepatitis serology (serology testing required at screening), as follows:
- Hepatitis B virus (HBV): Patients with positive serology for hepatitis B defined as positivity for hepatitis B surface antigen (HBsAg) or hepatitis B core antibody (anti-HBc).
- Hepatitis C virus (HCV): Patients with positive hepatitis C serology unless HCV- (RNA) is confirmed negative.
- HIV positive patients;
- Active fungal, bacterial, and/or viral infection that requires systemic therapy; Note: active controlled as well as chronic/recurrent infections are at risk of reactivation/infection during teatment with obinutuzumab and/or venetoclax);
- Vaccination with a live vaccine a minimum of 28 days prior to registration.
- Use of any other experimental drug or therapy within 28 days of baseline;
- Concurrent use of other anti-cancer agents or treatments;
- History of prior malignancy, except for conditions as listed below if patients have recovered from the acute side effects incurred as a result of previous therapy:
- Malignancies surgically treated with curative intent and with no known active disease present for ≥ 3 years before randomization
- Adequately treated non-melanoma skin cancer or lentigo maligna without evidence of disease
- Adequately treated cervical carcinoma in situ without evidence of disease
- Severe cardiovascular disease (arrhythmias requiring chronic treatment, congestive heart failure or symptomatic ischemic heart disease) (CTCAE grade III-IV, see appendix D);
- Severe pulmonary dysfunction (CTCAE grade III-IV, see appendix D);
- Severe neurological or psychiatric disease (CTCAE grade III-IV, see appendix D);
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, hypertension, hyperthyroidism or hypothyroidism etc.)
- Women who are pregnant or lactating;
- Fertile men or women of childbearing potential unless: (1). surgically sterile or ≥ 2
years after the onset of menopause (2). willing to use a highly effective contraceptive method (Pearl Index <1) such as oral contraceptives, intrauterine device, sexual abstinence or barrier method of contraception in conjunction with spermicidal jelly during study treatment and in female patients for 18 months after end of antibody treatment and male patients for 6 months after end of treatment.
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule.
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- Trial Online Process (TOP, https://www.hdc.hovon.nl/top). A logon to TOP can be requested at the HOVON Data Center for participants.
- By faxing the completed registration/randomization CRF +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31.10.7041560 Monday through Friday, from 09:00 to 17:00 CET
HOVON interim/final analyses for the coming 6 months: Final analysis induction treatment (EHA-2020; Q1-2020)
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
