GCLLSG HO140 CLL
Main info
- Identifier:
- HO140 CLL
- Sponsor:
- GCLLSG
- Working group party:
- CLL
- Age:
- >= 18
- Stage:
- 1st Line
- Echelon:
- Level D
- Included patients:
-
926(of 920)
- Active sites:
-
38(of 58)1 sites are pending
- Title:
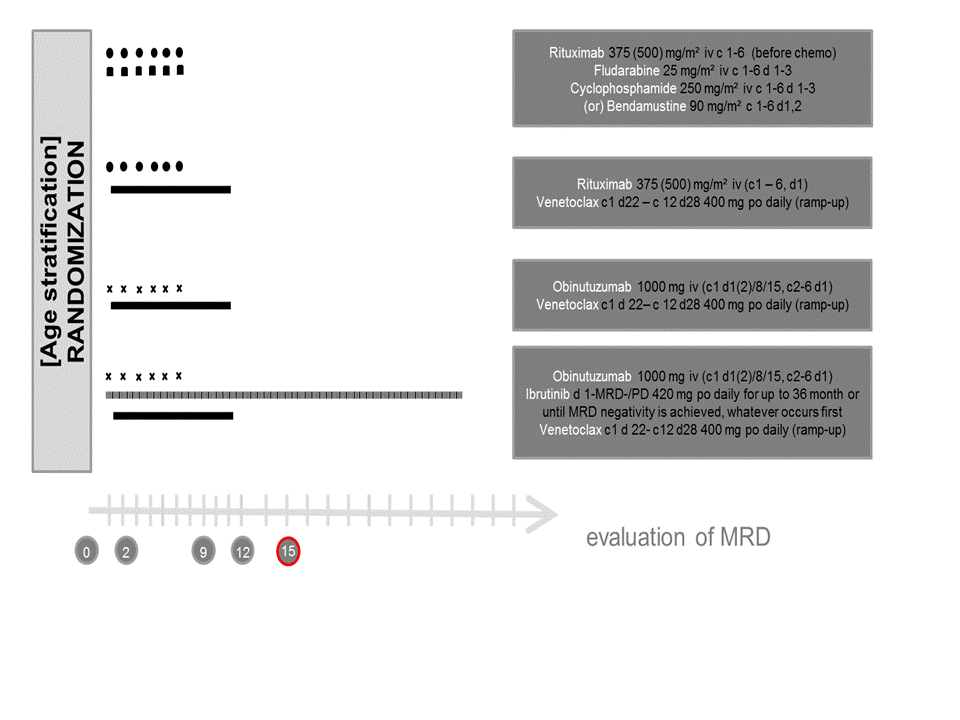
A Phase 3 multicenter, randomized, propective, open-label trial of standard chemoimmunotherapy (FCR/BR) versus Rituximab plus Venetoclax (RVE) versus
Obinutuzumab (GA101) plus Venetoclax GVE) versus Obinutuzumab plus Ibrutinib plus Venetoclax (GIVE) in fit patients with previously untreated chronic Lymphocytic Leukemia (CLL) without DEL(17P) or TP53 mutation.
Timeline
News
01-May-2019: Amendment 7 is approved for BE
It mainly concerns the update of the protocol (4.0). You can best download the zip file (found at section 'Belgium specific documentation'). In the Overview folders & files is stated which document is located at which directory and also indicates which documents are removed / added.
17-Apr-2019: Amendment 8 is approved for NL
It mainly concerns the update of the protocol (4.0). You can best download the HO140_ITF_NL_17APR2019.zip file (found at section 'other documents'). In the HO140_ITF_17APR2019_Overview folders & files.xlsx is stated which document is located at which directory and also indicates which documents are removed / added. UPDATE on 01MAY2019 - PLEASE SEE THE UPDATED VERSION OF THE ITF AND OVERVIEW FOLDERS AND FILES.
24-Apr-2018: Lab manual updated V04
A new lab manual has been provided by GCLLSG, version v04. You should have received new waybills for the HO140 trial from TNT. Also the shipment forms have been updated to v04. Please find the new instructions in the lab manual.
19-Apr-2018: New statement of expenses form
A new statement of expenses form for the HO140 study is available. In this new version de HepC RNA test is included. This compensation will (also retrospectively) apply for all included patients that have done this test.
22-Jan-2018: Training attendance log
The training attendance log of the Investigators Meeting has been added in the ITF. You can best download the HO140_ITF_BE_22JAN2018.zip or HO140_ITF_NL_22JAN2018.zip file. In the HO140_ITF overview folders en files_be_22JAN2018.xls or HO140_ITF overview folders en files_nl_22JAN2018.xls are stated which document is located at which directory and also indicates which documents are removed / added.
22-Jan-2018: Amendment 6 is approved for BE
It concerns a change in LI (Dr. Sylvia Snauwaert will take over the role of local investigator from Dr. Achiel van Hoof) at the site AZ St. Jan. You can best download the HO140_ITF_BE_22JAN2018.zip file (found at section 'Belgium specific documentation'). In the HO140_ITF overview folders en files_be_22JAN2018.xls is stated which document is located at which directory and also indicates which documents are removed / added.
11-Dec-2017: Amendment 3 is approved for BE
It mainly concerns the update of the protocol (3.0) and Mabthera will be provided as study medication for the FCR/BR arm as well. You can best download the HO140_ITF_BE_11DEC2017.zip file (found at section 'Belgium specific documentation'). In the HO140_ITF overview folders en files_be_11dec2017.xls is stated which document is located at which directory and also indicates which documents are removed / added.
05-Dec-2017:Central lab shipment forms updated
The TNT Express hotline number on the central lab shipment forms were adjusted. Please work closely with the Clinical Desk of TNT, so the arrival of the samples will not be delayed. The new version was sent to the sites and placed on the HOVON website and the ITF.
13-Nov-2017: Lab manual updated
Use the Clinical Desk of TNT to send samples to the central lab. Do not use the waybills send by GCLLSG, the clinical desk of TNT will create those for you. Find further information in the lab manual.
08-Nov-2017: CRF guidelines added.
There were previously some minimal guidelines on how to fill out the CRFs. These are now renewed. you can find them after logging in, at CRF instructions.
25-Oct-2017: Amendment 3 is approved for NL
It mainly concerns the update of the protocol (3.0) and Mabthera will be provided as study medication for the FCR/BR arm as well. You can best download the HO140_ITF_NL_25OCT2017.zip file (found at section 'other documents'). In the HO140_ITF_25OCT2017_Overview folders & files.xlsx is stated which document is located at which directory and also indicates which documents are removed / added.
20-Sep-2017: Correction of QoL (QLQ-C30) Dutch version.
By accident the version previously provided to the Dutch sites was still carrying the complete birthdate. The new version was send to the sites today and placed on the HOVON website and the ITF.
12-Sep-2017: Amendment 2 + 3 submission available for BE.
The local submission of amendment 02 and amendment 03 are now available for Belgium as download of a zip file in section 'Belgian specific documentation'. PLease submit both dossiers to your local EC.
12-Sep-2017: Amendment 2 is approved for NL.
It mainly concerns the update of the protocol and trial medication documents like Venetoclax patient diary, card and SPC. You can best download the HO140_ITF_NL_12SEP2017.zip file (found at section 'other documents'). In the HO140_ITF_12SEP2017_Overview folders & files.xlsx is stated which document is located at which directory and also indicates which documents are removed / added.
Please note that you can find all participating sites and whether or not they are activated in the section Registration & randomization of patients.
08-May-2017: Amendment 1 is approved for NL.
It mainly concerns an update of the ICF (now version 13MAR2017). Please send in this version when you wish to get activated.
02-Mar-2017: Study has been opened recently. You can best download the HO140_ITF_02MAR2017.ZIP file to have all documents at once. In the HO140_ITF_02MAR2017_Overview folders & files.xlsx is stated which document is located at which directory. Please let me know if there are any questions (p.cornelisse@erasmusmc.nl)
Flow
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Study Specific
- Objectives:
The primary objective of the study is to evaluate the efficacy of obinutuzumab (GA101) plus venetoclax (GVe) versus standard chemoimmunotherapy (BR/FCR) concerning MRD negativity measured by flow cytometry in peripheral blood (PB) approximately 15 months after start of therapy [MRD-Staging 3; MO15] and obinutuzumab plus ibrutinib plus venetoclax (GIVe) versus standard chemoimmuno-therapy (BR/FCR) concerning progression free survival (PFS) in previously untreated, fit CLL patients without del(17p) or TP53 mutation.
Secondary endpoints are efficacy parameters, particularly MRD negativity and PFS comparisons between
all four treatment arms.Exploratory objectives focus on the evaluation of relationship between various baseline markers and clinical outcome parameters but also on the correlation between MRD in BM and PB, as well as MRD in BM and PFS/EFS/OS and MRD in PB and PFS/EFS/OS. Moreover, comparisons of the outcome between FCR and BR will be considered.
Eligibility
- Inclusion Criteria:
- Documented CLL requiring treatment according to iwCLL criteria.
- Age at least 18 years.
- Life expectancy ≥ 6 months.
- Ability and willingness to provide written informed consent and to adhere to the study visit schedule and other protocol requirements.
- Adequate bone marrow function indicated by a platelet count >30 x10^9/l (unless directly attributable to CLL infiltration of the bone marrow, proven by bone marrow biopsy)
- GFR ≥70ml/min directly measured with 24hr urine collection, calculated according to the modified formula of Cockcroft and Gault (for men: GFR ≈ ((140 - age) x bodyweight) / (72 x creatinine), for womenvx 0, 85) or an equally accurate method.
- For patients with creatinine values within the normal range the calculation of the clearance is not necessary.
- Dehydrated patients with an estimated creatinine clearance less than 70 ml/min may be eligible if a repeat estimate after adequate hydration is > 70 ml/min.
- Adequate liver function as indicated by a total bilirubin≤ 2 x, AST/ALT ≤ 2.5 x the institutional ULN value, unless directly attributable to the patient’s CLL or to Gilbert’s Syndrome.
- Negative serological testing for hepatitis B (HBsAg negative and anti- HBc negative; patients positive for anti-HBc may be included if PCR for HBV DNA is negative and HBV-DNA PCR is performed every month until 12 months after last treatment cycle), negative testing for hepatitis C RNA within 6 weeks prior to registration.
- Eastern Cooperative Oncology Group Performance Status (ECOG) performance status 0-2.
- Exclusion Criteria:
- Any prior CLL-specific therapies (except corticosteroid treatment administered due to necessary immediate intervention; within the last 10 days before start of study treatment, only dose equivalents up to 20 mg prednisolone are permitted).
- Transformation of CLL (Richter‘s transformation).
- Decompensated hemolysis, defined as ongoing hemoglobin drop in spite of prednisolone or intravenous immunoglobulins (IVIG) being administered for hemolysis.
- Detected del(17p) or TP53 mutation.
- Patients with a history of PML.
- Any comorbidity or organ system impairment rated with a single CIRS (cumulative illness rating scale) score of 4 (excluding the eyes/ears/nose/throat/larynx organ system), a total CIRS score of more than 6 or any other life-threatening illness, medical condition or organ system dysfunction that, in the investigator´s opinion, could compromise the patients safety or interfere with the absorption or metabolism of the study drugs (e.g. inability to swallow tablets or impaired resorption in the gastrointestinal tract).
- Urinary outflow obstruction.
- Malignancies other than CLL currently requiring systemic therapies, not being treated with curative intent before (unless the malignant disease is in a stable remission due to the discretion of the treating physician) or showing signs of progression after curative treatment.
- Uncontrolled or active infection.
- Patients with known infection with human immunodeficiency virus (HIV).
- Requirement of therapy with strong CYP3A4 and CYP3A5 inhibitors/inducers.
- Anticoagulant therapy with warfarin or phenoprocoumon, (alternative anticoagulation is allowed (e.g. DOACs), but patients must be properly informed about the potential risk of bleeding under treatment with ibrutinib).
- History of stroke or intracranial hemorrhage within 6 months prior to registration.
- Use of investigational agents which might interfere with the study drug within 28 days prior to registration.
- Vaccination with live vaccines 28 days prior to registration.
- Major surgery less than 30 days before start of treatment.
- History of severe allergic or anaphylactic reactions to humanized or murine monoclonal antibodies, known sensitivity or allergy to murine products.
- Known hypersensitivity to any active substance or to any of the excipients of one of the drugs used in the trial.
- Pregnant women and nursing mothers (a negative pregnancy test is required for all women of childbearing potential within 7 days before start of treatment; further pregnancy testing will be performed regularly).
- Fertile men or women of childbearing potential unless:
- surgically sterile or ≥ 2 years after the onset of menopause
- Prisoners or subjects who are institutionalized by regulatory or court order.
- Persons who are in dependence to the sponsor or an investigator.
Registration Details
A central medical review of the screening documents including patient´s baseline characteristics (Physical and radiological examination results, blood count and serum chemistry as well as CIRS) and results from the central diagnostics (especially immunophenotyping for confirmation of CLL diagnosis and fluorescence in situ hybridization (FISH) as well as mutation analysis of CLL genes for exclusion of 17p deletion or TP53 mutation respectively) will be performed by GCLLSG study physicians in order
to verify the patient´s eligibility to the trial. Afterwards randomization will be performed centrally via IXRS (interactive voice and web response system). Please note: Approval of enrollment and notification of randomized arm by the GCLLSG central
study office is mandatory before initiation of study treatment.
At time of final MRD analysis (month 49), it is estimated that the time-point of the PFS interim analysis will be reached (after 65% of the total of 213 PFS events, i.e. 138 PFS events will trigger the interim analysis).
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
