GIMEMA HO142 CML
Main info
- Identifier:
- HO142 CML
- Sponsor:
- GIMEMA
- Working group party:
- CML/MPN
- Age:
- >= 18
- Stage:
- 2nd Line
- Echelon:
- Level C-HIC
- Included patients:
-
26(of 26)
- Active sites:
-
9(of 17)
- Title:
Sustained treatment-free remission in BCR-ABL+ chronic myeloid leukemia: a prospective study comparing Nilotinib versus Imatinib with switch to Nilotinib in absence of optimal response.
Timeline
News
11-02-2020: New SAE form v5_28-10-2019
9-10-2019: the HO142 was approved for the Belgium on 30 July 2019. All site documents have already been sent to the participating BE sites and on the website
06-03-2019: the lab manual and sample form have been adjusted. All samples intended for the central lab must be sent to the VUmc. See new version v09
21-12-2018: Study open, the first site in NL, Albert Schweitzer is open for inclusion.
FYI: Activation procedure in the HO142
Your hospital will be activated entirely through GIMEMA, the sponsor of the HO142. The procedure will be slightly different than what you are used to in a HOVON sponsored study. In this study there is study specific monitoring and a monitor (from the CRO Phidea ) will come along to do the initiation in your hospital. The activation procedure takes 2-3 weeks on average.
Flow
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Study Specific
- Objectives:
Primary Objective
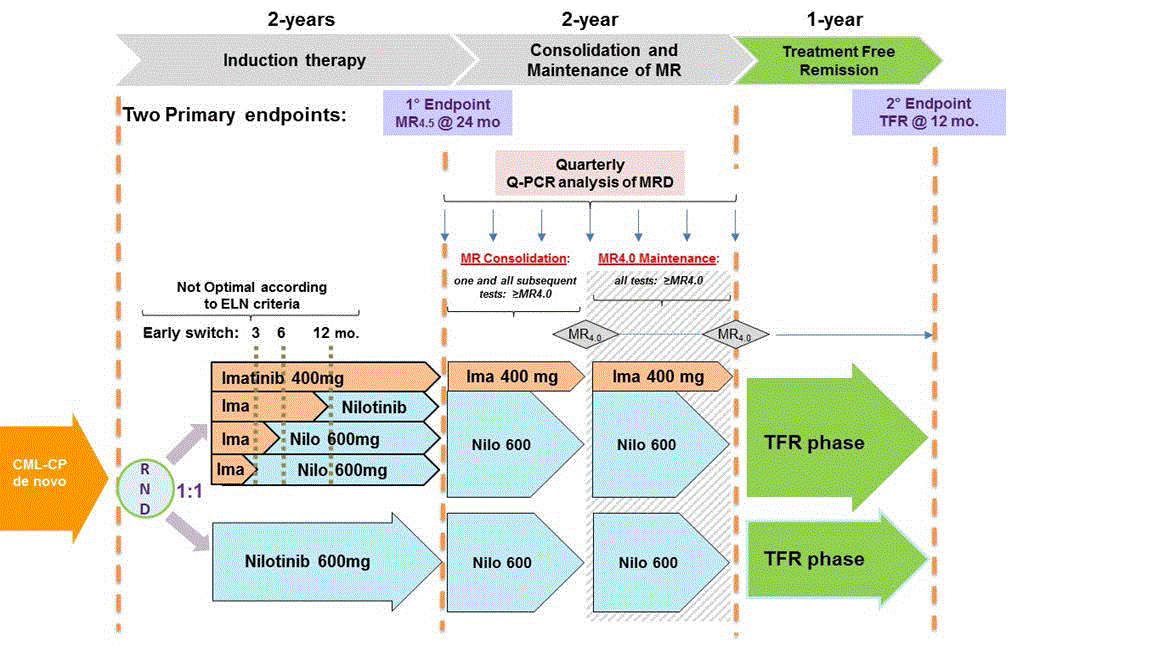
The study will investigate in newly diagnosed CP-CML patients the efficacy of NIL frontline therapy vs IM followed by switch to NIL in the case of absence of optimal response as defined by the ELN criteria using two primary end-points:
− To evaluate the rates of molecular response (MR4.5) at 24 months
− To evaluate the rate of patients who remain in sustained treatment free remission (≥MR3.0) without molecular relapse 12 months after entering the TFR phase. The molecular relapse is defined as loss of MMR, or confirmed loss of MR3.0.Secondary Objectives
1. Monitoring the molecular response.
2. Progression-free survival (PFS) in the two arms of the study.
3. Overall Survival in the two arms of the study.
4. Rates of major molecular response (MR3.0) during the study in the two arms of the study.
5. The dynamics of molecular response
6. The relationship between baseline characteristics and the primary objectives
7. The relationship between early molecular response and the primary objectives
8. To assess the safety profile of both arms.
9. To determine the rate and the time-distribution of the discontinuation causes of the first-line TKI.
10. To investigate quality of life (QoL) differences between treatment arms over time
Eligibility
- Inclusion Criteria:
Patients eligible for inclusion in this study have to meet all of the following criteria:
- Patients with a confirmed diagnosis of BCR/ABL+ CML in chronic phase
- Documented chronic phase CML must meet all the following criteria:
- < 15% blasts in peripheral blood
- < 30% blasts plus promyelocytes in peripheral blood
- < 20% basophils in the peripheral blood
- ≥ 100 x 109/L (≥ 100,000/mm3) platelets
- Age ≥18
- ECOG performance status of 0-2
- Evidence of typical BCR-ABL transcripts which are amenable to standardized RQ-PCR
- Adequate end organ function as defined by:
- Total bilirubin < 1.5 x ULN (ULN = upper limit of normal in a local institution lab). Does not apply to patients with isolated hyperbilirubinemia (e.g., Gilbert’s disease) grade < 3
- SGOT (AST) and SGPT (ALT) ≤ 3 x ULN
- Serum amylase and lipase ≤ 2 x ULN
- Alkaline phosphatase ≤ 2.5 x ULN
- Serum creatinine < 1.5 x ULN
- Written informed consent prior to any study procedures.
- Exclusion Criteria:
- Previous treatment with BCR-ABL inhibitors for more than 30 days.
- Expression of any atypical BCR-ABL transcripts, instead of the classical P210-encoding type with the e13a2 or the e14a2 junction at screening.
- Previous anticancer agents (hydroxyurea, anagrelide, interferon) for CML for more than three months.
- Poorly controlled diabetes mellitus (defined as HbA1c >8%)
- Prior documented history of coronary heart disease, including myocardial infarction, coronary bypass, coronary stent, and symptomatic angina as defined at page 30 in Exclusion Criteria.
- Uncontrolled hypertension
- History of peripheral arterial occlusive disease.
- History of acute pancreatitis within 12 months of study entry, or a past medical history of chronic pancreatitis.
- Patients actively receiving therapy with strong CYP3A4 inhibitors and/or inducers which cannot be either discontinued or switched to a different medication prior to starting study drug.
- Patients who are currently receiving treatment with any medications that have the potential to prolong the QT interval and for which cannot be either safely discontinued or switched to a different medication prior to starting study drug.
- Women of childbearing potential, defined as all women physiologically capable of becoming pregnant, unless they are using effective methods of contraception during dosing of study treatment.
- Patients unable to understand and to comply with study instructions and requirements.
- Refusal to give informed consent.
Registration Details
A patient can be registered after verification of eligibility criteria.
A patient has to be registered to the representative of the Study Management Committee (SMC) by filling the ELEGIBILITY FORM available on-line. This must be done prior to the start of the protocol treatment.
To contact the Study Management Committee:
For GIMEMA Sites:
Tel: 0817462037
Fax: 0817462165
Mail: sustrenim@gmail.com
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
