HOVON HO143 MM
Main info
- Identifier:
- HO143 MM
- Sponsor:
- HOVON
- Working group party:
- Myeloma
- Age:
- >= 18
- Stage:
- 1st Line
- Echelon:
- Level D
- Included patients:
-
133(of 132)
- Active sites:
-
49(of 50)
- Title:
Efficacy and tolerability of ixazomib, daratumumab and low dose dexamethasone (IDd) followed by ixazomib and daratumumab maintenance therapy until progression for a maximum of 2 years in unfit and frail newly diagnosed multiple myeloma patients; an open-label phase II trial.
Timeline
News
09-01-2019
*HOVON143 MM Pharmacy Info Letter vs 03 available.21-12 -2018
- HOVON143 MM Newsletter vs 4 available.
- Please send in your patient registration survey.
03-12 -2018 - FOR BELGIUM:
- HOVON143 MM ICF version VS6 15aug2018 available. From today this ICF document must be used.
- Please send in your local updated ICF version to the HDC for approval.
- See also email correspondence: HOVON143 ICF vs 6 activation in Belgium.msg
03-12-2018
- HOVON 143 MM FRAIL ARM of the trial:
- The required number of FRAIL patients has been reached.
- New FRAIL patients can no longer be registered in the HOVON143 trial.
- HOVON 143 MM UNFIT ARM of the trial:
- The required number of 66 UNFIT patients has almost been reached.
- There is a HOVON143 screening waiting list for the last 18 UNFIT patients. Only patients listed in this list will actually be registered by the HDC.
27-08-2018 - FOR BELGIUM:
- New Protocol VS4.0 and Pre-ICF VS 4 form available. From today these documents must be used.
- HOVON143 MM PRE- ICF version VS4 10juli2018
- HOVON143 protocol v4.0 17may2018
- Please send in your local updated Pre-ICF version to the HDC for approval.
- Please send original signed Local Investigator signature page to the HDC.
- See also email correspondence:
- HOVON143 MM Protocol vs. 4 en Pre-ICF v4 activation in Belgium.msg
- HOVON143 new Lab manual vs 7 and Imaging guidelines vs 3 activation in BELGIUM.msg (dd 27aug2018)
09-07-2018 - FOR THE NETHERLANDS ONLY:
- New Protocol, ICF and Pre-ICF forms available. From today these documents must be used.
- HOVON143 MM ICF version 3.2 17mei2018
- HOVON143 MM Pre-ICF version 2.2 17mei2018
- HOVON143 protocol v4.0 17may2018
- Please send in your local updated ICF and Pre-ICF versions to the HDC for approval.
- Please send original signed Local Investigator signature page to the HDC.
- See also email correspondence:
- HOVON143 Protocol en ICF amendement activatie in NEDERLAND.msg (dd 09jul2018)
13-03-2018
- The HOVON Data Center has a new postal address.
- Due to this change new versions of the QoL and Assessment CRFs were made (dd 05mar2018)
- Please use these new CRF versions to avoid loss of questionnaires.
- New version of the 'HO143 MM Instruction for IMWG Frailty Score calculation' is also available.
- HO143 MM Instruction for IMWG Frailty Score calculation vs 3 05mar 2018.pdf
- In this new version the link to the site for Frailty score calculation is changed.
04-01-2018
- New ICF and Pre-ICF forms available. From today these forms must be used.
- HOVON143 MM ICF version 3.1 16nov2017
- HOVON143 MM Pre-ICF form version 2.1 16nov2017
- Please send in your local updated ICF and Pre-ICF versions to the trial manager Sonia Cunha for approval.
- HOVON143 ICF amendement activatie en updated ABR form vs 9 dd 05-12-2017 n.a.v. site amendement.msg (dd 04jan2018)
- Updated ABR form version 9 05dec2017 available
- HOVON143 ICF v3.1 NL_16nov2017_Final_ for sites.doc
- HOVON143 pre-study ICF v2.1 16nov2017_Fnal for sites.docx
- HOVON143 cumulative-VUmc METC approvals vs 22dec2017.pdf
- HOVON143 cumulative-CA approvals vs 22dec2017.pdf
- HOVON143 ABR ABR-formulier voor dossier NL59458.029 vs 8 dd 16-11-2017_tracked changes.pdf
- HOVON143 ABR ABR-formulier voor dossier NL59458.029 vs 9 dd 05-12-2017signed.pdf
18-12-2017
- New SAE form version 02 available
- See also email correspondence:
- HOVON143 MM new SAE form vs 02 dd 04SEP2017.msg (dd 18-dec-2017)
- hovon143_sae-report-form_vs 02 04SEP2017.pdf
- Please use this form to report new SAEs
03-11-2017
- Transition to 400 mg daratumumab vials
- See also email correspondence:
- HOVON143 transition daratumumab from 100 mg to 400 mg vials in The Netherlands.msg (dd 03-nov-2017)
- The new version of the 400 mg daratumumab vials (vs 2 17-08-2017) received central approval
- Please use the 400 mg vials Daratumumab ressuply form for daratumumab orders.
- HOVON143 Daratumumab 400 mg vials_144-16-342 - Resupply Drug Order Request Form_v4.pdf
- HO143 MM Pharmacy info letter vs02_03nov2017.pdf
26-09-2017
- New version Statement of Expenses form NL vs 30-08-2017 available
- HOVON143 Statement of Expenses form NL 30-8-2017.pdf
07-09-2017
- The new version of the protocol (vs 3.1 dd 17.jul.2017) received central approval
- Please send in your signed local investigators protocol page.
- New study documents that can be downloaded from the HOVON website:
- HOVON143- pro v3.1 17JUL2017 -signed.pdf
- HOVON143- sheme of study vs 3.1, 17jul2017.pdf
- HOVON143- Protocol summary of changes vs 1 04sep2017.pdf
- HOVON143 cumulative-CA approvals vs 04sep2017.pdf
- HOVON143 cumulative-VUmc METC approvals vs 04sep2017.pdf
- HO143 Study drug accountability form_Daratumumab vs 2.0.pdf
- HO143 Study drug accountability form_Ixazomib vs 2.0.pdf
31-08-2017:
- Study documents available: ITF labels and Drug Administration/toedienings_protocol:
- HOVON143_ITF_Table of Contents_v1.0_24aug2017.docx;
- HOVON143_ITF_spine label_v1.0_24aug2017.docx;
- HOVON143_ITF_labels_v1.0_24aug2017.doc;
- HOVON143_ITF_toelichting_lokaal_v2_28feb2017.pdf
- HOVON143_Toedienings_Moederprotocol_ UMCA vs 3.0 30aug2017.pdf
Flow
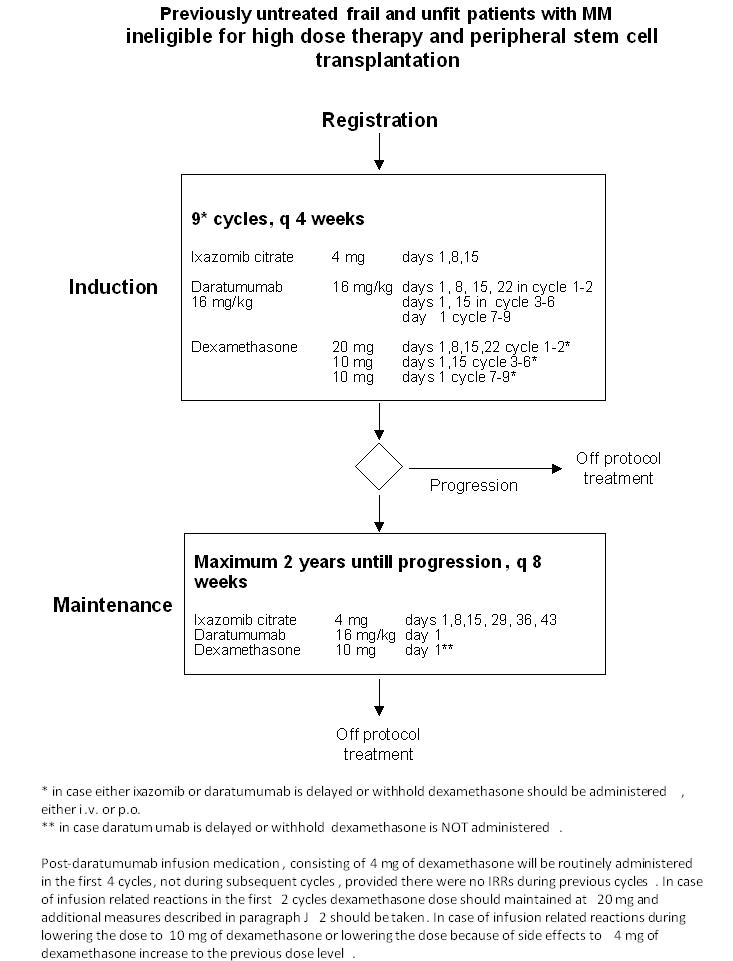
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
Primary objective:
- To determine the efficacy, defined as overall response rate (ORR), of 9 cycles of ixazomib, daratumumab and low dose dexamethasone. Overall response will be defined as (stringent) complete response ((s)CR), very good partial (VGPR) response and partial response (PR)
Secondary objectives:
- To determine the tolerability, defined as discontinuation rate due to treatment related toxicity, of 9 cycles of ixazomib, daratumumab and low dose dexamethasone
- To determine adverse events of CTCAE grade 2-4
- To determine complete response (CR) and very good partial response (VGPR) after 9 induction cycles
- To determine complete response (CR) and very good partial response (VGPR) on protocol
- To determine immunophenotypic complete response after 9 induction cycles
- To determine immunophenotypic complete response on protocol
- To determine the flow Minimal Residual Disease negative complete remission
- To determine the imaging plus flow MRD negative complete remission
- To determine progression free survival (PFS)
- To determine overall survival (OS)
- To determine efficacy of therapy determined as time to response and the time to best response
- To determine the effect of maintenance therapy with ixazomib and daratumumab in terms of improvement of response during maintenance
- To determine the tolerability of maintenance therapy, defined as discontinuation rate due to treatment related toxicity of ixazomib and daratumumab
- To determine time to next treatment
- To determine PFS2
- To evaluate quality of life (QoL)
Exploratory objectives:
- To identify geriatric assessment outcomes that predict feasibility and the toxicity of treatment
- To identify biological markers; sarcopenia and senescence markers, that reflect biological age and that predict feasibility and the toxicity of treatment
- To identify immunological and molecular prognostic markers that predict outcome and toxicity
- To identify biomarkers for response
- To investigate the prognostic value of Minimal Residual Disease
- To investigate the prognostic value of FDG-PET-CT at diagnostics and in follow up
Eligibility
- Inclusion Criteria:
- Previously untreated patients with a confirmed diagnosis of multiple myeloma according to IMWG criteria (see protocol appendix A);
- Measurable disease according to the IMWG criteria (see protocol appendix A); (If plasmacytoma is the only measurable parameter, the patient is not allowed to be included in the study, because of difficult response evaluation)
- Patients who are either unfit or frail according to the IMWG criteria (see protocol appendix B);
- Age 18 years or older;
- Absolute neutrophil count (ANC) ≥ 1.0 x109/l and platelet count ≥ 75x109/l; Platelet transfusions and G-CSF to help patients meet eligibility criteria are not allowed;
- Written informed consent, including consent for additional bone marrow and blood sampling and a skin biopsy (with the understanding that consent may be withdrawn by the patient at any time without consequences to future medical care);
- Patient is capable of giving informed consent;
- Negative pregnancy test at study entry (only for women of childbearing potential);
- Male patients and female patients of childbearing potential must agree to use adequate contraception from the time of signing the informed consent form through 90 days after the last dose of study drug (see protocol section 9.4 for details).
- Exclusion Criteria:
- Non-secretory MM;
- Plasma cell leukemia;
- Systemic Amyloid Light-chain (AL) amyloidosis;
- Central nervous system involvement;
- Known allergy to any of the study medications, their analogues, or excipients in the various formulations of any agent;
- Neuropathy, grade 1 with pain or grade ≥ 2;
- Severe cardiac dysfunction (NYHA classification III-IV, appendix D);
- Screening 12-lead ECG showing a baseline QT interval as corrected by Fridericia’s formula (QTcF) >470 msec;
- Chronic obstructive pulmonary disease (COPD) with an Forced Expiratory Volume in 1 second (FEV1) < 50% of predicted normal. Note that FEV1 testing is required for patients suspected of having COPD and subjects must be excluded if FEV1 <50% of predicted normal;
- Moderate or severe persistent asthma within the past 2 years or currently uncontrolled asthma of any classification. (Note that subjects who currently have controlled intermittent asthma or controlled mild persistent asthma are allowed in the study);
- Significant hepatic dysfunction (total bilirubin ≥ 3 x ULN or transaminases ≥ 5 times normal level) except patients with Gilbert’s syndrome as defined by > 80% unconjugated bilirubin;
- Creatinine clearance <20 ml/min or Calculated Glomerular Filtration Rate [ml/min/1.73m2] <20;
- Patients with active, uncontrolled infections;
- Patients known to be Human Immunodeficiency Virus (HIV)-positive;
- Patients seropositive for hepatitis B, defined by a positive test for hepatitis B surface antigen [HBsAg]. Patients with resolved infection (ie, subjects who are HBsAg negative but positive for antibodies to hepatitis B core antigen [anti-HBc] and/or antibodies to hepatitis B surface antigen [anti-HBs]) must be screened using real-time polymerase chain reaction (PCR) measurement of hepatitis B virus (HBV) DNA levels. Those who are PCR positive will be excluded. EXCEPTION: Subjects with serologic findings suggestive of HBV vaccination (anti-HBs positivity as the only serologic marker) AND a known history of prior HBV vaccination, do not need to be tested for HBV DNA by PCR.
- Patients seropositive for hepatitis C (except in the setting of a sustained virologic response [SVR], defined as aviremia at least 12 weeks after completion of antiviral therapy).
- Known GI disease or GI procedure that could interfere with the oral absorption or tolerance of ixazomib including difficulty swallowing;
- Active malignancy other than MM requiring treatment or a malignancy that has been treated with chemotherapy currently affecting bone marrow capacity;
- Systemic treatment, within 14 days before the first dose of ixazomib, with strong CYP3A inducers (rifampin, rifapentine, rifabutin, carbamazepine, phenytoin, phenobarbital), or use of St. John’s wort;
- Pre-treatment with cytostatic drug, immunomodulatory drugs (IMiDs) or proteasome inhibitors. Radiotherapy (provided the involved field is small and there are ≥ 7 days between radiotherapy and administration of ixazomib) or a short course of steroids (e.g. 4 day treatment of dexamethasone 40 mg/day or equivalent) are allowed;
- Major surgery within 14 days before enrollment;
- Any serious medical or psychiatric illness, or familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule;
- Participation in other clinical trials, including those with other investigational agents not included in this trial, within 30 days of the start of this trial and throughout the duration of this trial;
- Female patients who are lactating.
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- Trial Online Process (TOP, https://www.hdc.hovon.nl/top). A logon to TOP can be requested at the HOVON Data Center for participants.
- By faxing the completed registration CRF +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31.10.7041560 Monday through Friday, from 09:00 to 17:00 CET
Planned HOVON interim/final analyses for the coming 6 months: final analyis unfit patients, 9 induction cycles (ASH-2020; Q2-2020)
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
