Aarhus University Hospital HO144 NHL
Main info
- Identifier:
- HO144 NHL
- Sponsor:
- Aarhus University Hospital
- Working group party:
- Lymphoma
- Age:
- 18-85
- Stage:
- 2nd Line
- Echelon:
- Level D
- Included patients:
-
4(of 30)
- Active sites:
-
9(of 10)1 sites are pending
- Title:
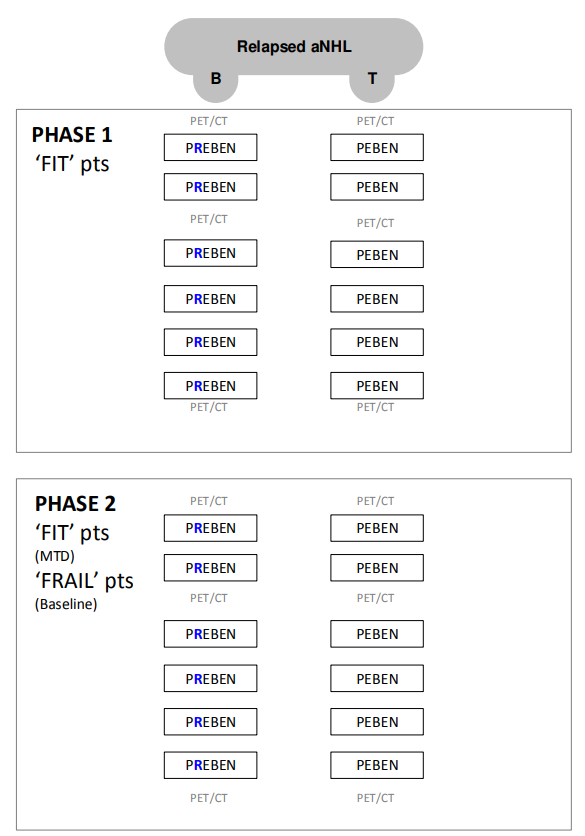
A phase 1/2 study of combination therapy with pixantrone , (rituximab for CD20 positive tumours), etoposide and bendamustine in patients with relapsed aggressive lymphoma of B or T cell phenotype – P[R]EBEN.
Timeline
Flow
Details
- Phase:
- Prospective Phase I/II study
- Monitoring Type:
- Not any more
- Objectives:
Primary Objectives
- Determine the MTD of pixantrone, rituximab (only in CD20 positive tumors), etoposide, and bendamustine in ‘fit' patients with rel aNHL of B- or T-cell phenotype.
- Evaluate the ORR and PFS using the combination of pixantrone, rituximab (only in CD20 positive tumors), etoposide, and bendamustine either at the identified MTD (P[R]EBEN-fit) in ‘fit’ patients or at the baseline dose level (P[R]EBEN-frail) in ‘frail’ patients with rel aNHL.
Secondary objectives
- Evaluate the CR, PR, duration of response, and OS using the combination of pixantrone, rituximab (only in CD20 positive tumors), etoposide, and bendamustine in patients with Bor T-cell NHL.
- Evaluate the safety and tolerability of combination therapy with pixantrone, rituximab (only in CD20 positive tumors), etoposide, and bendamustine in patients with aggressive B- or T-cell NHL.
- To perform molecular analyses at nucleic acid (DNA, RNA, microRNA) and protein level to see if specific molecular features can predict responder versus non-responder status.
Eligibility
- Inclusion Criteria:
- Patients with a histologically confirmed relapse of an aggressive lymphoma of T- or B-cell phenotype (including follicular lymphoma grade 3b). For excluded histological entities see ‘Exclusion Criteria’
- Phase 1 + Phase 2 ‘fit’ patients:
- Age 18-70 years at the time of inclusion
- ECOG performance score (PS) 0-1 at protocol entry (for ECOG definition see appendix A)
- Deemed ‘fit’ by the treating physician
- Phase 2 ‘frail’ patients:
- Age 71-85 years at the time of inclusion, and/or
- ECOG PS 2-3 at protocol entry (for ECOG definition see appendix A), and/or
- Deemed ‘frail’ by the treating physician
- At least 6 months response duration since last given course of treatment
- Estimated life expectancy of 3 months or longer
- Measurable disease
- Hemoglobin ≥ 8 g/dL (≥5 mmol/l) (can be post transfusion)
- Platelets ≥ 100 x 10^9/L; ≥ 75 x10^9/ permitted if bone marrow involvement
- Absolute neutrophil count ≥ 1.5 x 10^9/L; ≥ 1.0 x 10^9/L permitted if documented bone marrow involvement
- Serum bilirubin ≤ 1.5 x upper limit of normal (ULN); patients with proven Gilbert’s syndrome (≤ 5 x ULN) may be enrolled.
- Serum glutamic-oxaloacetic transaminase (AST) and/or serum glutamic-pyruvic transaminase (ALT) ≤ 2.5 x ULN, or ≤ 5 x ULN if elevation is due to hepatic involvement by lymphoma
- Serum creatinine ≤ 2 x ULN
- Women of childbearing potential must use safe anticonception (e.g. contraceptive pills, intrauterine devices etc.) during the study and 12 months after the last administration of study drugs
- Male patients must use contraception for the duration of the study and 6 months after the last administration of study drugs if his partner is of childbearing potential
- Written informed consent.
- Exclusion Criteria:
- Patients with primary refractory disease (e.g. progressing under platinum-containing or similar salvage therapy) defined as < 6 months response duration from last given course of treatment.
- High-dose therapy with autologous stem cell rescue within the last 6 months prior to study entry.
- Following T-cell lymphoma entities:
- T-cell lymphoblastic lymphoma
- Hepatosplenic T-cell lymphoma
- Extranodal NK/T, nasal type
- Subcutaneous panniculitis-like
- Primary cutaneous T-cell lymphoma
- Primary leukemic T-cell lymphoma
- Following B-cell lymphoma entities:
- Transformed indolent B-cell lymphomas
- Post-transplant B-cell lymphoproliferative disease
- HIV-associated B-cell lymphoma
- Concurrent severe and/or uncontrolled medical disease which is not lymphoma-related
- Left ventricular ejection fraction (LVEF) < 45%
- Suspected or documented central nervous system involvement by NHL
- Patients known to be antigen positive for HIV and/or hepatitis B and/or hepatitis C
- Patients with active, uncontrolled infections
- Vaccination with live, attenuated vaccines within 4 weeks of inclusion
- Pregnant and/or breastfeeding women
- History of active cancer during the past 5 years, except basal carcinoma of the skin or stage 0 cervical carcinoma
- Known hypersensitivity to one or more of the study drugs
- Unwillingness or inability to comply with the protocol
Registration Details
Patients should be registered using a study specific patient registration form at the CTO:
Helle Erbs Toldbod
Department of Hematology, A-CTO
Palle Juul-Jensens Boulevard 99
DK-8200 Aarhus N
Denmark
Tel.: +45 7845 5855
E-Mail: a-cto@auh.rm.dk
The following information will be registered at study entry:
- Institution name
- Name of responsible investigator
- Sex
- Date of birth
- Date of primary lymphoma diagnosis
- Histological lymphoma subtype at primary diagnosis
- Date of present histologically confirmed relapse
- Histological lymphoma subtype at present relapse
- Eligibility criteria (i.e. all inclusion and exclusion criteria)
Each patient will be given a unique patient study number by the CTO. For phase 1 patients, the CTO will inform the site of which dose level to apply.
Q3 2021
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
