GU Frankfurt HO145 AML
Main info
- Identifier:
- HO145 ETAL-4
- Sponsor:
- GU Frankfurt
- Working group party:
- Leukemia
- Age:
- 18-70
- Stage:
- 2nd Line
- Echelon:
- Level A
- Included patients:
-
12(of 165)
- Active sites:
-
4(of 18)
- Title:
European Intergroup Trial on panobinostat maintenance after HSCT for high-risk AML and MDS - A randomized, multicenter phase III study to assess the efficacy of panobinostat maintenance therapy vs. standard of care following allogeneic stem cell transplantation in patients with high-risk AML or MDS (ETAL-4 / HOVON-145).
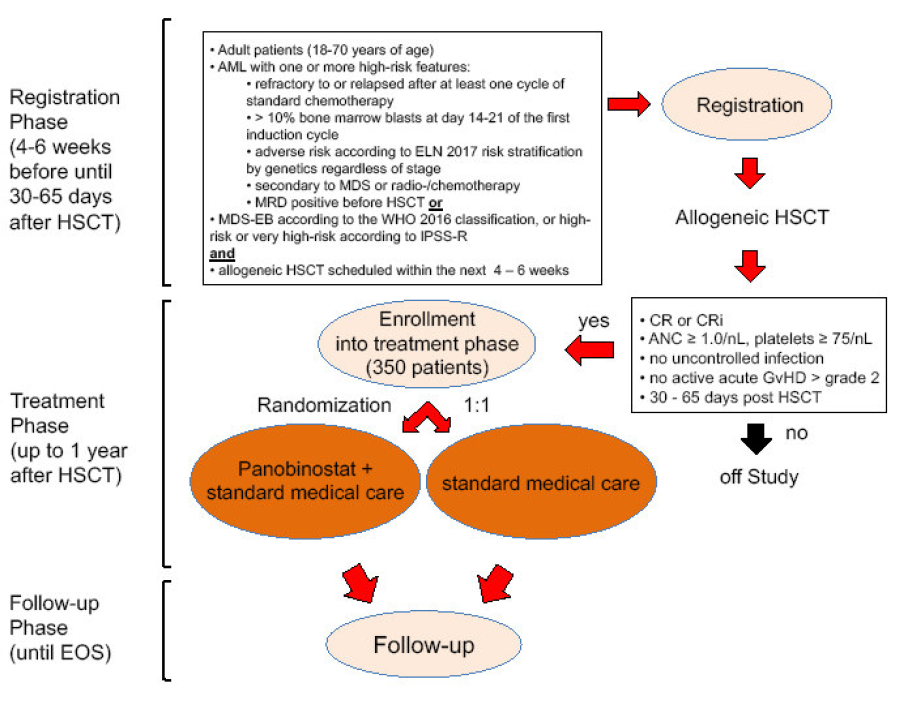
Timeline
Flow
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Not any more
- Objectives:
Primary objective
- To determine the efficacy of panobinostat maintenance therapy versus standard of care administered to patients with high-risk MDS or AML in complete hematologic remission after an allogeneic hematologic stem cell transplantation (HSCT)
Secondary objectives
- To assess the safety and tolerability of panobinostat maintenance therapy after HSCT compared with standard of care
- To evaluate HRQoL of patients under panobinostat maintenance therapy after HSCT
- To study the treatment effect in subgroups of patients defined by treatment approach (i.e. HOVON-approach vs. RIC vs MAC conditioning), donor type (HLA-compatible versus haploidentical) and molecular distinct subgroups of AML/MDS.
Eligibility
- Inclusion Criteria:
Inclusion criteria for registration prior to HSCT:
- Adult patients (18-70 years of age)
- AML (except acute promyelocytic leukemia with PML-RARA and AML with BCRABL1) according to WHO 2016 classification (Appendix 1) with high-risk features defined as one or more of the following criteria:
- refractory to or relapsed after at least one cycle of standard chemotherapy
- > 10% bone marrow blasts at day 14-21 of the first induction cycle
- adverse risk according to ELN 2017 risk stratification by genetics (Appendix 2) regardless of stage
- secondary to MDS or radio-/chemotherapy
- MRD positive before HSCT based on flow cytometry or PCR
or
- MDS with excess blasts (MDS-EB) according to the WHO 2016 classification (Appendix 3), or high-risk or very high-risk according to IPSS-R (Appendix 4)
and
- First allogeneic HSCT scheduled within the next 4-6 weeks using one of the following donors, conditioning regimens (Appendix 5) and strategies for GvHD prophylaxis:
- a. Matched sibling or matched unrelated donor (i.e. 10/10 or 9/10 HLA-matched) or haploidentical family donor
- b. Conditioning regimens:
- # Reduced-intensity conditioning:
- ## Fludarabine/Melphalan
- ## Fludarabine/Busulfan2 (FB2)
- # Myeloablative conditioning:
- ## Fludarabine/Busulfan4 (FB4)
- ## Busulfan/Cyclophosphamide (BU/CY)
- ## Fludarabine/TBI 8 Gy
- ## Cyclophosphamide/TBI 12 Gy
- # Fludarabine/Cyclophosphamide/TBI 2 Gy in combination with post-Tx cyclophosphamide (TP-CY) only
- # Thiotepa/Busulfan/Fludarabine (TBF) in the context of an haploidentical HSCT only
- # In case of active disease at HSCT, salvage chemotherapy prior to conditioning is permitted
- c. Strategies for GvHD prophylaxis:
- # HLA-matched donors:
- ## CSA + MMF +/- ATG
- ## CSA + MTX +/- ATG
- ## PT-CY + CSA
- # Haploidentical donors:
- ## PT-CY + CSA + MMF
- No history of significant cardiac disease and absence of active symptoms, otherwise documented left ventricular EF ≥ 40%
- Written informed consent for registration
Inclusion criteria for enrollment after HSCT
- Adult patients with high-risk AML or MDS as defined above
and
- First allogeneic HSCT performed within 30 - 65 days prior to enrollment
- Eastern Cooperative Group (ECOG) performance status ≤ 2 (Appendix 6)
- Complete hematologic remission or complete hematologic remission with incomplete recovery (see section 14.1) documented by bone marrow aspiration within 14 days prior to enrollment
- Laboratory test results maximum 14 days prior to enrollment within the following ranges:
- Absolute neutrophil count ≥ 1.0 x 10^9/L
- Platelet count ≥ 75 x 10^9/L
- Potassium, magnesium and phosphorus within normal limits
- Serum creatinine clearance ≥ 30 mL/min
- Total bilirubin ≤ 1.5 x ULN
- AST (SGOT) and ALT (SGPT) ≤ 2.5 x ULN
- Negative serum pregnancy test (within 14 days prior to enrollment) in women of child-bearing potential (WOCBP): A woman is considered of childbearing potential, i.e. fertile, following menarche and until becoming postmenopausal unless permanently sterile. Permanent sterilization methods include hysterectomy, bilateral salpingectomy and bilateral oophorectomy. A postmenopausal state is defined as no menses for 12 months without an alternative medical cause.
- Willingness of WOCBP to use a highly effective method of contraception during study treatment and for three months following the last dose of study drug. Highly effective methods of contraception include:
- oral, intravaginal or transdermal combined (estrogen and progestogen containing) hormonal contraceptive associated with inhibition of ovulation plus barrier contraceptive
- oral, injectable or implantable progestogen-only hormone contraception associated with inhibition of ovulation plus barrier contraceptive
- intrauterine hormone-releasing system (IUS) plus barrier contraceptive
- intrauterine device (IUD)
- bilateral tube occlusion
- vasectomised partner: Vasectomised partner is a highly effective method of contraception provided that partner is the sole sexual partner of the WOCBP trial participant and that the vasectomised partner has received medical assessment of the surgical success.
- sexual abstinence: Sexual abstinence is considered a highly effective method only if defined as refraining from heterosexual intercourse during study treatment and for three months following the last dose of study drug, and if it is the preferred and usual lifestyle of the WOCBP trial participant. Women using hormonal contraceptives should additionally use a barrier method of contraception (preferably male condom).
- Willingness of male subjects whose sexual partners are WOCBP to use a highly effective method of contraception as defined above during the man’s treatment and for six months following the last dose of study drug.
- Written informed consent.
- Exclusion Criteria:
Exclusion criteria for registration prior to HSCT:
- Prior treatment with a DAC inhibitor
- Hypersensitivity to the active substance or to any of the excipients of panobinostat
- HIV or HCV antibody positive
- Psychiatric disorder that interferes with ability to understand the study and give informed consent, and/or impacts study participation or follow-up.
- Female patients who are pregnant or breast feeding
- History of another primary malignancy that is currently clinically significant or currently requires active intervention
Exclusion criteria for enrollment after HSCT:
- Active acute GvHD grade III-IV according to modified Glucksberg criteria (Appendix7)
- Active acute GvHD grade II or chronic GvHD moderate/severe according to NIH criteria (Appendix 8) requiring systemic corticosteroids > 0.5 mg/kg body weight of methylprednisolone equivalent or combination immunosuppressive treatment
- Uncontrolled or significant heart disease, including recent myocardiac infarction, cardiac failure (NYHA II-IV), unstable angina pectoris, or clinically significant bradycardia
- Long QT syndrome
- QTcF ≥480 msec on screening ECG to be performed within 14 days prior to enrollment
- Concurrent use of medications that have a relative risk of prolonging QT interval or of inducing Torsade de Pointes, if such treatment cannot be discontinued or switched to a different medication prior to the first dose of study drug (see Table 9).
- Other concurrent severe and/or uncontrolled medical conditions (e.g., uncontrolled diabetes mellitus, chronic obstructive or chronic restrictive pulmonary disease including dyspnoea at rest from any cause) or history of serious organ dysfunction or disease involving the heart, kidney, or liver and/or seropositive HIV or HCV.
- Serious active infection
- CMV reactivation, which is not responsive to first-line valganciclovir or ganciclovir
- Impaired gastrointestinal (GI) function or GI disease that may significantly alter the absorption of oral panobinostat (e.g., ulcerative disease, uncontrolled nausea, vomiting, diarrhea, malabsorption syndrome, obstruction, or stomach and/or small bowel resection).
Registration Details
Registration of patients via the eCRF provided by the sponsor.
https://ctcn.kgu.de/apps/WebObjects/ST21-productive-
DataCapture.woa/wa/choose?customer=HAEM1
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
