HOVON HO148 AML
Archived
Main info
- Identifier:
- HO148 AML
- Sponsor:
- HOVON
- Working group party:
- SCT & Supportive care
- Age:
- 18-70
- Stage:
- 2nd Line
- Echelon:
- Level A
- Included patients:
-
12(of 40)
- Active sites:
-
5(of 10)
- Title:
A phase I/II feasibility study of the combination of panobinostat and midostaurin in recipients of allogeneic stem cell transplantation with Flt3-ITD AML.
Timeline
Scheduled
Actual
2016
01 Jan
Opportunity
2017
15 Nov
EC Approval
2017
01 Dec
First Site
2017
22 Dec
Submission in Progress
2018
08 Jan
FPI
2018
14 May
EC Approval
2018
17 Jul
Activated
2018
17 Jul
First Site
2018
02 Aug
FPI
2020
12 Mar
ClosedForInclusionActualStart
2021
18 May
Endpoint Analysis
2021
30 Jun
Endpoint Analysis
2021
30 Jun
CloseoutInProgressLastPtOutActualStart
2024
02 Mar
Archived
2025
05 Mar
CloseoutInProgressLastPtOutScheduledStart
2039
30 Jun
Destruction
News
please note: No need to send in MRD samples to central lab anymore due to early closure.
12-03-2020: The HO148 is closed for inclusion of patients.
Flow
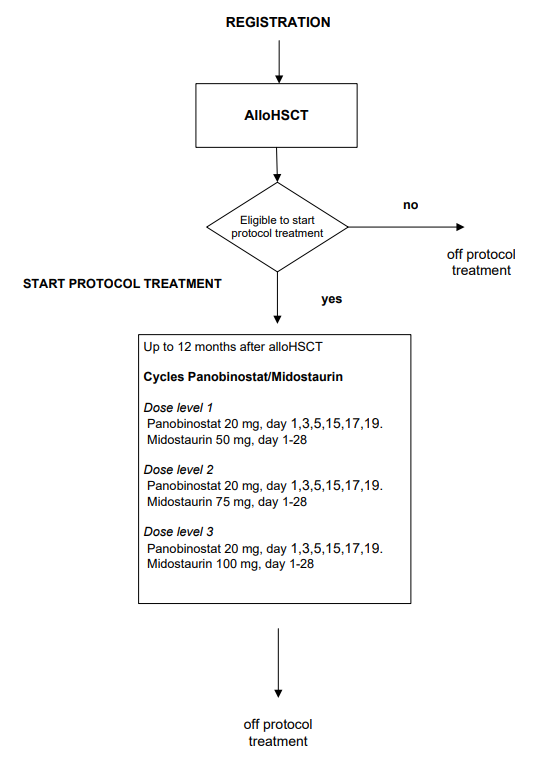
Details
- Phase:
- Prospective Phase I/II study
- Monitoring Type:
- Not any more
- Objectives:
Primary objective:
- To assess the safety and feasibility of post-transplant panobinostat combined with midostaurin in patients with adverse risk AML/RAEB with FLT3-ITD with high allelic ratio in terms of dose limiting toxicity.
Secondary objectives:
- To assess feasibility in terms of completion of the protocol treatment
- To assess efficacy in terms of:
- Complete hematological remission (with full peripheral blood recovery) rate at 3, 6 and 12 months post alloHSCT.
- Immunological remission (residual disease assessed by multicolor flowcytometry) at 6 months post alloHSCT
- Relapse/progression rate as assessed after cycle 1, 3, 5 and 7 and at 12 months post alloHSCT, or at approx. 3, 6 and 12 months post alloHSCT in case of early termination of protocol treatment.
- Overall survival (OS) from alloHSCT
- Progression free survival (PFS) from alloHSCT with relapse (for patients in CR) and progression (for patients in PR) and death from any cause as events
- Engraftment and chimerism at 3, 6 and 12 months post alloHSCT
- To assess toxicity in terms of:
- The incidence and nature of (serious) adverse events
- The incidence and severity of acute and chronic GvHD up to 12 months post alloHSCT
- NRM up to 12 months post alloHSCT
- Number and percentage of registered patients starting protocol treatment
- Number and percentage of patients receiving post-transplant epigenetic therapy after alloHSCT
Eligibility
- Inclusion Criteria:
- Adult patients (18-70 years of age)
- AML (except acute promyelocytic leukemia, AML M3 and bcr/abl positive AML) according to WHO 2016 classification (Appendix A) or RAEB with IPSS-R ≥ 1.5 with high mutant to wild-type allelic ratio of FltFLT3-ITD
- First allogeneic HSCT scheduled within the next 2 months upon having achieved hematological remission (< 5% blasts at the bone marrow level)
- Matched sibling or matched unrelated donor (i.e. 10/10 or 9/10 HLA-matched) or haploidentical donor
- Using one of the following conditioning regimens:
- Fludarabine/Cyclophosphamide/TBI 2 Gy in combination with post-Tx cyclophosphamide (TPT-CY) only
- Fludarabine/Busulfan or Melphalan/Fludarabine/TBI or fludarabin/TBI 8 Gy with post-transplant cyclophosphamide.
- Strategies for GvHD prophylaxis:
- HLA-matched donors:
- PT-CY + CSA
- Haploidentical donors:
- PT-CY + CSA + MMF
- No history of significant cardiac disease and absence of active symptoms, otherwise documented left ventricular EF > 40%
- Negative serum pregnancy test for female patients of childbearing potential, at registration
- Female patients of childbearing potential must use an effective contraceptive method during the study and for a minimum of 6 months after study treatment
- Written informed consent
- Exclusion Criteria:
- Known HIV or HCV positivity
- History of active malignancy during the past 2 years with the exception of basal carcinoma of the skin or carcinoma “in situ” of the cervix or breast
- Pregnant or breast-feeding female patients
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- By ALEA; Use goto eCRF button > select the [Patient tab] and click the [Add new patient] button. Complete all items and click the [Submit] button
- By faxing the completed registration/randomization CRF +31 (0)10 704 1028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31 (0)10 704 1560 Monday through Friday, from 09:00 to 17:00 CET
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
Site
5 results
Order by
Accrual rate
Activation date
NL-Rotterdam-ERASMUSMC
5
17 Jul 2018
NL-Amsterdam-VUMC
3
18 Dec 2018
NL-Groningen-UMCG
3
29 May 2019
NL-Amsterdam-AMC
1
04 Dec 2018
NL-Maastricht-MUMC
09 Jan 2019
