HOVON HO150 AML
Main info
- Identifier:
- HOVON 150 AML_AMLSG 29-18
- Sponsor:
- HOVON
- Working group party:
- Leukemia
- Age:
- >= 18
- Stage:
- 1st Line
- Echelon:
- Level C-HIC&C-SCT
- Included patients:
-
974(of 971)
- Active sites:
-
191(of 219)1 sites are pending
- Title:
A phase 3, multicenter, double-blind, randomized, placebo-controlled study of ivosidenib or enasidenib in combination with induction therapy and consolidation therapy followed by maintenance therapy in patients with newly diagnosed acute myeloid leukemia or myelodysplastic syndrome with excess blasts-2, with an IDH1 or IDH2 mutation, respectively, eligible for intensive chemotherapy.
Timeline
News
17SEP2024 End of inclusion IDH1 cohort
Please be informed that as of 17SEP2024, the IDH1 cohort has completed the inclusion.
12APR2023 End of inclusion IDH2 cohort
Please be informed that as of 11APR2023, the IDH2 cohort has completed the inclusion. As of today it is not possible to include patients in the IDH2 cohort.
We thank you for your support in this cohort and for your continued support in the IDH1 cohort, which we expect to still be open for approximately one more year from now.
News 02 June 2022: The IDH2 cohort accrual will reopen at 07 JUN 2022
11MAR2022 New lab manual
The new lab manual has been sent to all participating sites.
03 March 2022: Updated CRF instructions are available.
News 07APR2021: implementation Amendment 02
As of April 7th, 2021, Amendment 02 has been implemented. The following documents have been updated on the website:
- Protocol v5, 15JUN2020
- Protocol signature page, v5
- Template ICF v4, 15JUN2020
- Screening ICF, 09MAY2020
- Statement of Expenses form
- Pharmacy Manual, 31MAR2021
- Lab Manual, 11FEB2021
- REGRAND form, 25JUN2020
Update October 2019
The following documents have been updated after release:
- Site lab manual (current date 01OCT2019), reason for update: further clarification on sending of samples.
Flow
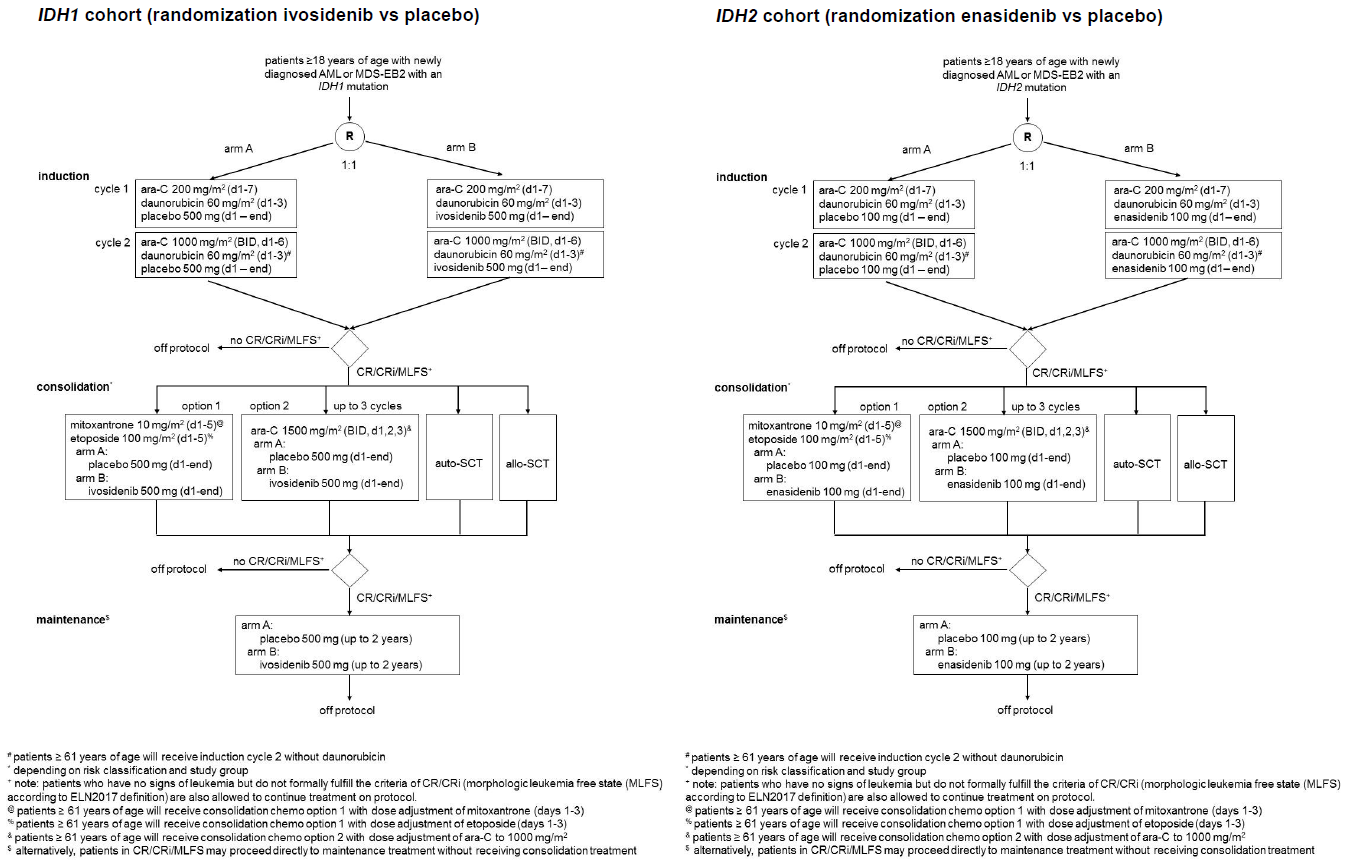
Details
- Phase:
- Prospective double-blind randomized phase III study
- Monitoring Type:
- Study Specific
- Objectives:
Primary study objective:
- To compare event-free survival (EFS) between ivosidenib/enasidenib and placebo in combination with induction therapy and consolidation therapy followed by maintenance therapy in patients with newly diagnosed AML or MDS-EB2, with an IDH1 or IDH2 mutation, eligible for intensive chemotherapy.
Secondary study objectives:
- Key secondary study objective:
- To determine if treatment with ivosidenib/enasidenib, as compared to placebo prolongs overall survival (OS).
- Other secondary study objectives:
- To compare relapse-free survival (RFS), cumulative incidence of relapse (CIR) and cumulative incidence of death (CID) after CR/CRi between treatment including ivosidenib/enasidenib and treatment including placebo.
- To evaluate minimal residual disease (MRD) status at sequential time points throughout treatment and CRMRD− rate between treatment including ivosidenib/enasidenib vs. placebo, using molecular and/or flow cytometric techniques.
- To assess the safety and tolerability of treatment including ivosidenib/enasidenib vs. placebo by comparing the frequency and severity of adverse events according to CTCAE.
- To compare complete remission (CR/CRi) rates for treatment including ivosidenib/enasidenib vs. placebo.
- To assess the time to hematopoietic recovery (ANC 0.5 and 1.0x109/l; platelets 50 and 100x109/l) after each chemotherapy treatment cycle.
- To determine quality of life (QoL) during maintenance treatment with ivosidenib/enasidenib vs. placebo.
- Exploratory objective:
- To study the pharmacokinetics of treatment including ivosidenib/enasidenib in a small subset of patients.
See protocol section 13 for endpoint definitions and appendices B and C for definitions of response criteria and outcome measures.
Eligibility
- Inclusion Criteria:
- Age ≥18 years
- Newly diagnosed AML or MDS-EB2 defined according to WHO criteria, with a documented IDH1 or IDH2 gene mutation (as determined by the clinical trial assay) at a specific site (IDH1 R132, IDH2 R140, IDH2 R172). AML may be secondary to prior hematological disorders, including MDS, and/or therapy-related (in which prior disease should have been documented to have existed for at least 3 months). Patients may have had previous treatment with hypomethylating agents (HMAs) for MDS. HMAs have to be stopped at least four weeks before registration
- Patients with dual mutant FLT3 and IDH1 or IDH2 mutations may be enrolled only if, for medical or other reasons, treatment with a FLT3 inhibitor is not considered.
- Considered to be eligible for intensive chemotherapy.
- ECOG/WHO performance status ≤ 2
- Adequate hepatic function as evidenced by:
- Serum total bilirubin ≤ 2.5 × upper limit of normal (ULN) unless considered due to Gilbert’s disease (e.g. a mutation in UGT1A1) (only for patients in IDH2 cohort), or leukemic involvement of the liver – following written approval by the (Co)Principal Investigator.
- Aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP) ≤ 3.0 × ULN, unless considered due to leukemic involvement of the liver, following written approval by the Principal Investigator.
- Adequate renal function as evidenced by creatinine clearance > 40 mL/min based on the Cockroft-Gault formula for glomerular filtration rate (GFR).
- Able to understand and willing to sign an informed consent form (ICF).
- Written informed consent
- Female patient must either:
- Be of nonchildbearing potential:
- Postmenopausal (defined as at least 1 year without any menses) prior to screening, or
- Documented surgically sterile or status posthysterectomy (at least 1 month prior to screening)
- Or, if of childbearing potential,
- Agree not to try to become pregnant during the study and for 6 months after the final study drug administration
- And have a negative urine or serum pregnancy test at screening
- And, if heterosexually active, agree to consistently use highly effective* contraception per locally accepted standards in addition to a barrier method starting at screening and throughout the study period and for 6 months after the final study drug administration.
Highly effective forms of birth control include:
- Consistent and correct usage of established hormonal contraceptives that inhibit ovulation,
- Established intrauterine device (IUD) or intrauterine system (IUS),
- Bilateral tubal occlusion,
- Vasectomy (A vasectomy is a highly effective contraception method provided the absence of sperm has been confirmed. If not, an additional highly effective method of contraception should be used.)
- Male is sterile due to a bilateral orchiectomy.
- Sexual abstinence is considered a highly effective method only if defined as refraining from heterosexual activity during the entire period of risk associated with the study drug. The reliability of sexual abstinence needs to be evaluated in relation to the duration of the clinical study and the preferred and usual lifestyle of the patient.
List is not all inclusive. Prior to enrollment, the investigator is responsible for confirming patient will utilize highly effective forms of birth control per the requirements of the CTFG Guidance document ‘Recommendations related to contraception and pregnancy testing in clinical trials’, September 2014 (and any updates thereof) during the protocol defined period.
- Female patient must agree not to breastfeed starting at screening and throughout the study period, and for 2 months and 1 week after the final study drug administration.
- Female patient must not donate ova starting at screening and throughout the study period, and for 6 months after the final study drug administration.
- Male patient and their female partners who are of childbearing potential must be using highly effective contraception per locally accepted standards in addition to a barrier method starting at screening and continue throughout the study period and for 4 months and 1 week after the final study drug administration
- Male patient must not donate sperm starting at screening and throughout the study period and for 4 months and 1 week after the final study drug administration.
- Subject agrees not to participate in another interventional study while on treatment.
- Exclusion Criteria:
- Prior chemotherapy for AML or MDS-EB2 (with the exception of HMA). Hydroxyurea is allowed for the control of peripheral leukemic blasts in patients with leukocytosis (e.g., white blood cell [WBC] counts > 30x109/L).
- Dual IDH1 and IDH2 mutations.
- Acute promyelocytic leukemia (APL) with PML-RARA or one of the other pathognomonic variant fusion genes/chromosome translocations.
- Blast crisis after chronic myeloid leukemia (CML).
- Known allergy or suspected hypersensitivity to Ivosidenib or Enasidenib and/or any exipients.
- Taking medications with narrow therapeutic windows with potential interaction with investigational medication (see protocol Appendix I), unless the patient can be transferred to other medications prior to enrolling or unless the medications can be properly monitored during the study.
- Taking P-glycoprotein (P-gp) or breast cancer resistance protein (BCRP) transporter-sensitive substrate medications (see protocol Appendix J) unless the patient can be transferred to other medications within ≥ 5 half-lives prior to administration of ivosidenib or enasidenib, or unless the medications can be properly monitored during the study.
- Breast feeding at the start of study treatment.
- Active infection, including hepatitis B or C or HIV infection that is uncontrolled at randomization. An infection controlled with an approved or closely monitored antibiotic/antiviral/antifungal treatment is allowed.
- Patients with a currently active second malignancy. Patients are not considered to have a currently active malignancy if they have completed therapy and are considered by their physician to be at < 30% risk of relapse within one year. However, patients with the following history/concurrent conditions are allowed:
- Basal or squamous cell carcinoma of the skin
- Carcinoma in situ of the cervix
- Carcinoma in situ of the breast
- Incidental histologic finding of prostate cancer
- Significant active cardiac disease within 6 months prior to the start of study treatment, including New York Heart Association (NYHA) Class III or IV congestive heart failure (protocol appendix G); myocardial infarction, unstable angina and/or stroke; or left ventricular ejection fraction (LVEF) < 40% by ultrasound or MUGA scan obtained within 28 days prior to the start of study treatment.
- QTc interval using Fridericia’s formula (QTcF) ≥ 450 msec or other factors that increase the risk of QT prolongation or arrhythmic events (e.g., heart failure, family history of long QT interval syndrome). Prolonged QTc interval associated with bundle branch block or pacemaking is permitted with written approval of the (co) Principal Investigator.
- Taking medications that are known to prolong the QT interval (see Appendix K), unless deemed critical and without a suitable alternative. In those cases, they may be administered, but with proper monitoring (see section 10.2, Table 13).
- Dysphagia, short-gut syndrome, gastroparesis, or other conditions that limit the ingestion or gastrointestinal absorption of orally administered drugs.
- Clinical symptoms suggestive of active central nervous system (CNS) leukemia or known CNS leukemia. Evaluation of cerebrospinal fluid (CSF) during screening is only required if there is a clinical suspicion of CNS involvement by leukemia during screening.
- A known medical history of progressive multifocal leukoencephalopathy (PML).
- Immediately life-threatening, severe complications of leukemia such as uncontrolled bleeding, pneumonia with hypoxia or shock, and/or severe disseminated intravascular coagulation
- Any other medical condition deemed by the Investigator to be likely to interfere with a patient’s ability to give informed consent or participate in the study.
- Any psychological, familial, sociological or geographical condition potentially hampering compliance with the study protocol and follow-up schedule.
Registration Details
- Patients who are possibly eligible for this trial should first be screened to determine the IDH1/2 mutation status. This can only be done after the patient has provided a written informed consent for the screening. Screening is done through a separate screening database. There are two screening databases for this trial, one managed by HOVON and one managed by AMLSG. Each group/institute that is participating in this trial is assigned one of the two screening databases.
- Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- By ALEA; Use goto eCRF button > select the [Patient tab] and click the [Add new patient] button. Complete all items and click the [Submit] button
- By faxing the completed registration/randomization CRF +31 (0)10 704 1028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31 (0)10 704 1560 Monday through Friday, from 09:00 to 17:00 CET
The first Interim Analysis will be performed when the first 50 patients have either completed two cycles of induction therapy or discontinued the induction treatment.
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
