HOVON HO152 NHL
Main info
- Identifier:
- HO152 NHL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- >= 18
- Stage:
- 1st Line
- Echelon:
- Level D
- Included patients:
-
97(of 97)
- Active sites:
-
24(of 24)
- Title:
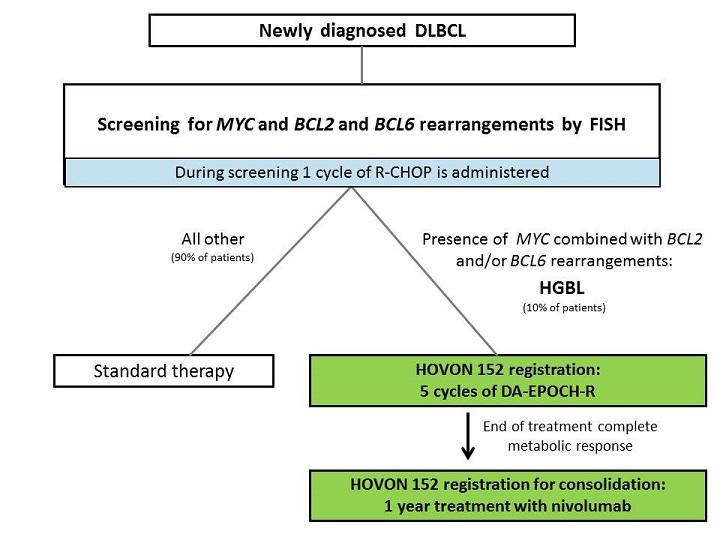
A phase II study evaluating the effect of DA-EPOCH-R induction followed by nivolumab consolidation in patients with newly diagnosed high grade B cell lymphoma (HGBL) with MYC and BCL2 and/or BCL6 rearrangements.
Timeline
News
As per March 23, 2022 the HO152 study is closed for inclusion of new patients.
Flow
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
Primary objective
- To increase 12 months DFS of DH/TH-HGBL patients in CMR after DA-EPOCH-R from 70% to 85% with nivolumab consolidation treatment.
Secondary objectives
- To evaluate CMR rate after completion of DA-EPOCH-R
- To evaluate 18 months PFS and OS of all patients
- To evaluate 12 months overall survival under consolidation (OSc) of patients registered for consolidation.
- To evaluate safety of nivolumab treatment
- To explore the accuracy of mid-treatment 18F-FDG PET-CT to predict CMR end-of-treatment.
- To explore the efficacy of nivolumab with regard to induction of MRD negativity by circulating tumor DNA and extracellular vesicle associated microRNA.
- To explore PD1/PDL1 expression in relation to NGS, GEP and to outcome
- To explore T cell subsets and clonality during treatment and in relation to MRD.
Eligibility
- Inclusion Criteria:
Inclusion criteria for DA-EPOCH-R induction
- High-grade B-cell lymphoma, with MYC in combination with BCL2 and/or BCL6 rearrangements as assessed by FISH according to the WHO 2016 classification including high-grade B-cell lymphoma with MYC and BCL2 rearrangements, transformed from previously untreated FL.
- Age ≥ 18 year.
- Patient started with or has received one course of full dose R-CHOP or DA-EPOCH-R (cycle 1). Starting with DA-EPOCH-R in cycle 1 is only allowed when FISH results (confirming DH/TH diagnosis) are directly available at diagnosis. [Reversed R-CHOP (cyclophosphamide, vincristine and doxorubicin on day 5) is allowed; local radiation or short course (max 7 days) of steroids (max 100 mg/day) before R-CHOP is allowed. Mini-R-CHOP is not allowed].
- WHO performance status 0-3 during or after induction cycle 1 (see appendix C).
- Ann Arbor stage II-IV at diagnosis (see appendix A).
- F-FDG PET scan and contrast enhanced CT-scan performed preferably within 28 days before start first induction cycle. When the PET or CT-scan is performed outside the 28-day window, consultation and written approval by the Principal Investigator for potential patient inclusion are necessary.
- Measurable disease: on contrast enhanced CT-scan at least 1 lesion/node with a long axis of >1.5 cm and at least one 18F-FDG avid lesion.
- Negative pregnancy test at study entry.
- Patient is willing and able to use adequate contraception until 6 months post last treatment administration.
- Written informed consent.
- Patient is capable of giving informed consent
Inclusion criteria for nivolumab consolidation
- Complete metabolic response on end of induction 18F-FDG PET-CT assessed with the Deauville response criteria (see 10.3) (or higher when due to inflammatory response, only possible after written approval by the Principal Investigator).
- Patient has completed treatment of at least 5 induction cycles.
- Exclusion Criteria:
Exclusion criteria for induction with DA-EPOCH-R
- All histopathological diagnoses other than DH/TH-HGBL (like testicular large B-cell lymphoma or primary mediastinal B-cell lymphoma) according to WHO 2016 classification.
- Known history of indolent lymphoma previously treated with immunochemotherapy.
- Inadequate renal function or creatinine clearance < 30 mL/min (after rehydration). Creatinine clearance may be calculated by Cockcroft –Gault formula: CrCl = (140 - age [in years]) x weight [kg] (x 0.85 for females)/ (0.815 x serum creatinine [μmol/L])
- Inadequate hepatic function: bilirubin > 3 times ULN (total) except patients with Gilbert's syndrome as defined by > 80% unconjugated bilirubin.
- Inadequate hematological function: ANC <1.0x10^9/L or platelets < 75x10^9 /L before induction cycle 1 unless lymphoma related.
- CNS localization of the lymphoma. CSF analysis before start of treatment is only necessary in case of suspicion of CNS localization.
- Female subject pregnant or breast-feeding.
- History of active malignancy during the past 5 years with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma.
- Active symptomatic ischemic heart disease, myocardial infarction, or congestive heart failure within the past year. In case of cardiac history, an echo or MUGA should be obtained and LVEF should exceed 40% to be eligible.
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, infection, hypertension, cancer, etc.) that would jeopardize the patient's ability to receive the regimen with reasonable safety.
- HIV positivity.
- Active Hepatitis B or C infection as defined by positive serology and transaminitis. Non-active Hepatitis B carriers may be included if protected (see 9.2.3).
- Severe pulmonary dysfunction (CTCAE grade III-IV, see appendix D).
- Subjects with active, known or suspected autoimmune disease. Subjects with vitiligo, type I diabetes mellitus, residual hypothyroidism due to autoimmune condition only requiring hormone replacement, psoriasis not requiring systemic treatment, or conditions not expected to recur in the absence of an external trigger are permitted to enroll.
- Subjects with a condition requiring systemic treatment with either corticosteroids (> 10 mg daily prednisone equivalents) or other immunosuppressive medications within 14 days of study drug administration. Inhaled or topical steroids, and adrenal replacement doses > 10 mg daily prednisone equivalents are permitted in the absence of active autoimmune disease.
- Prior treatment with an anti-PD1, anti-PDL1, anti-PDL2, or anti-CTLA-4 antibody, or any other antibody or drug specifically targeting T-cell costimulation or immune checkpoint pathways.
- Severe neurological or psychiatric disease.
- Current participation in another clinical trial interfering with this trial.
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule.
- Claustrophobia precluding PET-CT.
Exclusion criteria for nivolumab consolidation
- Inadequate renal function or creatinine clearance <30 mL/min (after rehydration). Creatinine clearance may be calculated by Cockcroft –Gault formula: CrCl = (140 - age [in years]) x weight [kg] (x 0.85 for females)/(0.815 x serum creatinine [μmol/L])
- Inadequate hepatic function: bilirubin > 3 times ULN (total) except patients with Gilbert's syndrome as defined by > 80% unconjugated bilirubin.
- Subjects with active, known or suspected autoimmune disease. Subjects with vitiligo, type I diabetes mellitus, residual hypothyroidism due to autoimmune condition only requiring hormone replacement, psoriasis not requiring systemic treatment, or conditions not expected to recur in the absence of an external trigger are permitted to enroll.
- Subjects with a condition requiring systemic treatment with either corticosteroids (> 10 mg daily prednisone equivalents) or other immunosuppressive medications within 14 days of study drug administration. Inhaled or topical steroids, and adrenal replacement doses > 10mg daily prednisone equivalents are permitted in the absence of active autoimmune disease.
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at the HOVON Data Center by one of the following options:
- By ALEA; select the [patient] tab and click the [ Add new patient] button. Complete all items and click the [Submit] button
- By faxing the completed registration/randomization CRF +31.10.7041028 Monday through Friday, from 09:00 to 17:00 CET
- By phone +31.10.7041560 Monday through Friday, from 09:00 to 17:00
Registration in ALEA should be done before starting the DA-EPOCH-R induction treatment. In addition to inclusion and exclusion criteria, lab values for eligibility are also requested.
NOTE: Second registration in ALEA before start of Nivolumab consolidation treatment. It is important to check whether a patient is eligible for inclusion in the consolidation phase.
Primary endpoint analysis (Q1/Q2 2024)
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
