HOVON HO165 CLL
Main info
- Identifier:
- HOVON 165 CLL
- Sponsor:
- HOVON
- Working group party:
- CLL
- Age:
- >= 18
- Stage:
- 2nd Line
- Echelon:
- Level D
- Included patients:
-
50(of 100)
- Active sites:
-
19(of 25)6 sites are pending
- Title:
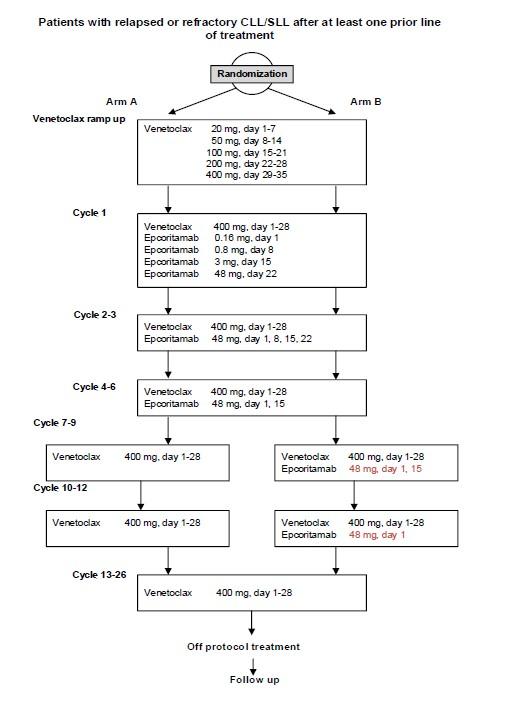
A prospective randomized phase I/II trial of venetoclax treatment (26 cycles) with 6 cycles or 12 cycles of epcoritamab in patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma
Timeline
News
16-Dec-2025
We have updated the central lab manual, please find the latest version of the manual and the shipment form in the download section.
08-Jan-2025
Inclusion for Phase II opened on 07-Jan only at the 4 sites that participated in phase I.
10-Apr-2024
The first site (DK-Rigshospitalet) has been activated today.
16-Jan-2024
The study was approved by all participating member states in CTIS.
12-Sep-2023
The study is submitted in CTIS.
Flow
Details
- Phase:
- Prospective randomized Phase I/II study
- Monitoring Type:
- HOVON Monitoring Visit
- Objectives:
Phase I
Primary Objective
To determine the RP2D for epcoritamab when used in combination with venetoclax for the phase II part of the study.Phase II
Primary Objective
To evaluate efficacy of venetoclax treatment (26 cycles) in combination with 6 cycles or 12 cycles of epcoritamab in patients with relapsed or refractory CLL in terms of undetectable minimal residual disease <10-4 (uMRD4) in the bone marrow (BM) for the two treatment groups separately.Secondary Objectives
- To evaluate the efficacy of epcoritamab in combination with venetoclax in terms of MRD depth in peripheral blood (PB),progression free survival (PFS), overall survival (OS), event free survival (EFS), overall response rate (ORR), time to next treatment (TTNT) for the two treatment groups separately.
- To evaluate the safety and tolerability of epcoritamab in combination with venetoclax.
- To evaluate quality of life (QoL) with epcoritamab in combination with venetoclax.
- To evaluate the incidence, severity, and type of infections.
Eligibility
- Inclusion Criteria:
- Documented relapsed or refractory CLL or SLL (SLL in phase II part only) following at least one systemic 1st-line treatment
- Requiring treatment according to IWCLL criteria (appendix A);
- Age at least 18 years;
- ECOG/WHO performance status 0-2;
- Adequate BM function defined as:
- Hemoglobin >5.6 mmol/l or Hb > 9 g/dL, unless low Hb is directly attributable to CLL/SLL infiltration of the BM, proven by BM biopsy;
- Absolute neutrophil count (ANC) >1.0 x 109/L (1,000/μL), unless low ANC is directly attributable to CLL/SLL infiltration of the BM, proven by BM biopsy;
- Platelet count >30 x 109/L (30,000/μL), unless low platelets is directly attributable to CLL/SLL infiltration in the BM;
- Estimated Glomerular Filtration Rate (eGFR) (MDRD) or estimated creatinine clearance (CrCl) ≥ 50ml/min (Cockcroft-Gault appendix 0);
- Adequate liver function as indicated:
- Serum aspartate transaminase (ASAT) and alanine transaminase (ALAT) ≤ 3.0 x upper limit of normal (ULN);
- Bilirubin ≤1.5 x ULN (unless bilirubin rise is due to Gilbert's syndrome or controlled autoimmune hemolytic anemia);
- Prothrombin time (PT)/International normal ratio (INR) <1.5x ULN and activated partial thromboplastin time (aPTT) <1.5 x ULN; unless receiving anticoagulation;
- Negative serological testing for hepatitis B virus (HBV) (Hepatitis B surface antigen (HBsAg) negative and hepatitis B core antibody (anti-HBc) negative) and hepatitis C virus (hepatitis C antibody). Patients who are positive for anti-HBc or hepatitis C antibody may be included if they have a negative PCR within 6 weeks before enrollment. Those who are PCR positive will be excluded; Please note: For patients positive for anti-HBc or antibodies for hepatitis C at screening but negative with retesting, HBV-DNA or HCV-DNA PCR, respectively, has to be repeated every month until 12 months after last dose of study treatment;
- Patient is able and willing to adhere to the study visit schedule and other protocol requirements;
- Patient is capable of giving informed consent;
- Written informed consent.
- Exclusion Criteria:
- Patient received treatment with anti-cancer agent as follows: a. Standard agents within 2 weeks or 5 half-lives, whichever is shorter, prior to the planned first dose of epcoritamab (excluding anti-CD20 mAbs and BTKi, which can be administered until first full dose of epcoritamab);
OR b. Patient received treatment with an investigational drug, within 4 weeks or 5 half-lives, whichever is shorter, prior to the planned first dose of Venetoclax;
- Prior treatment with a CD3 × CD20 bispecific antibody or CAR T-cell therapy
- Patient received prior venetoclax treatment within 24 months of registration OR patient had progressed during previous venetoclax treatment
- Transformation of CLL (Richter’s transformation);
- Prior allogeneic stem cell transplantation and/or solid organ transplantation;
- Patient with a history of confirmed progressive multifocal leukoencephalopathy (PML);
- Malignancies other than CLL/SLL currently requiring systemic therapy or not treated in curative intention or showing signs of progression after curative treatment;
- Known allergy to xanthine oxidase inhibitors and/or rasburicase;
- History of drug-specific hypersensitivity or anaphylaxis to any study drug (including active product or excipient components);
- Active bleeding or uncontrolled severe bleeding diathesis (e.g., hemophilia or severe von Willebrand disease);
- a. Ongoing active bacterial, viral, fungal, mycobacterial, parasitic or other infection requiring systemic treatment (excluding prophylactic treatment) at the time of enrollment or within the previous 2 weeks prior to the planned first dose of trial drug, including COVID-19 infection. Note that a past COVID-19 infection may be a risk factor, but if resolved and the subject is vaccinated, it may be allowable to enroll the subject.
b. Has suspected active or inadequately treated latent tuberculosis;
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled: infection, auto-immune hemolysis, immune thrombocytopenia, diabetes, hypertension, hyperthyroidism or hypothyroidism etc.);
- Patient known to be HIV-positive;
- Patient requiring treatment with a strong cytochrome P450 (CYP) 3A inhibitor/inducer (see appendix I);
- CTCAE grade III-IV cardiovascular disease including but not limited to:
- Unstable or uncontrolled disease/condition related to or affecting cardiac function, eg, unstable angina, congestive heart failure grade III or IV as classified by the New York Heart Association (see appendix A), uncontrolled clinically significant cardiac arrhythmia (CTCAE grade II or higher), or clinically significant electrocardiogram (ECG) abnormalities.
- Myocardial infarction within 6 months prior to registration.
- Patient age ≥75 and 2 or more active grade ≥2 cardiovascular conditions.
- Screening 12-lead ECG showing a baseline QT interval as corrected by Fridericia’s formula (QTcF) >480 msec. NOTE: this criterion does not apply to subjects with a left bundle branch block.
- Stroke or intracranial hemorrhage within 6 months prior to registration.
- Severe pulmonary dysfunction (CTCAE grade III-IV, see appendix D);
- Severe neurological or psychiatric disease (CTCAE grade III-IV, see appendix D);
- Neuropathy > CTCAE grade II
- Patient who has difficulty with or are unable to swallow oral medication, or have significant gastrointestinal disease that would limit absorption of oral medication;
- Vaccination with live vaccines within 28 days prior to registration;
- Major surgery within 28 days prior to registration;
- Pregnant women and nursing mothers;
- Fertile men or women of childbearing potential (WOCBP) unless: (1) surgically sterile or ≥ 2 years after the onset of menopause; (2) willing to use a highly effective contraceptive method such as oral contraceptives, intrauterine device or sexual abstinence during study treatment and for 12 months after last dose of epcoritamab and 30 days after last dose of venetoclax;
- Previous participation in the HO139 CLL or HO140 CLL trial and eligible for and willing to participate in the HO159 CLL trial;
- Current participation in other clinical trial and using study medication;
- Any psychological, familial, sociological and geographical condition potentially hampering compliance with the study protocol and follow-up schedule.
Registration Details
Eligible patients should be registered before start of treatment. Patients need to be registered at HOVON by one of the following options:
- HOVON registration database. See the HOVON website (www.hovon.nl) for the current link to ALEA Account for registration and a registration manual can be requested at HOVON.
- By sending the completed registration CRF by e-mail to hovon@erasmusmc.nl, Monday through Friday, from 09:00 to 17:00 CET
- By phone +31.10.7041560 Monday through Friday, from 09:00 to 17:00 CET
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Sex
- Age
- Date written informed consent
- Specific items patient gives consent for (see ICF for biobank)
- Eligibility criteria
All eligibility criteria will be checked.
Randomization will be performed by using random blocks. The block sizes will be chosen in such a way that the total number of 90 CLL patients that are treated at the RP2D will be balanced across the two treatment groups at the end of the trial, i.e., both in the phase 1 part and in the phase 2 part. In the phase 2 part separate blocks will be used for SLL patients so that the balance of CLL patients is guaranteed.
Each patient will be given a unique patient study number (a sequence number by order of enrolment in the trial). Patient study number will be given immediately by the online registration database or phone and confirmed by email.
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
