HOVON HO24 MM
Archived
Main info
- Identifier:
- HO24 MM / OG 96-020
- Sponsor:
- HOVON
- Age:
- 18-65
- Stage:
- 1st Line
- Included patients:
-
453
- Active sites:
-
0(of 39)38 sites are pending
- Title:
Myelo-Ablative Chemo-/ Radiotherapy and Autologous Stem Cell Transplantation as compared to only Chemotherapy in Patients with Multiple Myeloma: A Prospective Randomized Phase III Study.
Timeline
Scheduled
Actual
1995
15 Nov
Activated
2000
01 Apr
CloseoutInProgressLastPtOutActualStart
2010
01 Apr
Archived
2035
01 Apr
Destruction
Flow
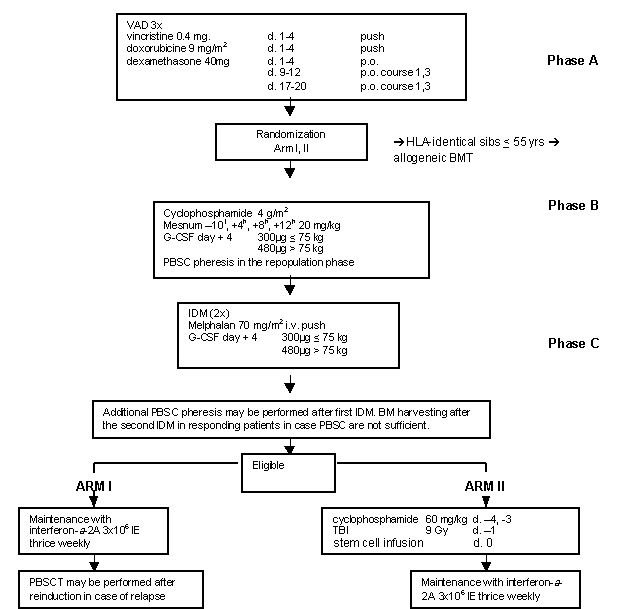
Details
- Phase:
- Prospective randomized Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Patients under 66 years of age with previously untreated Multiple Myeloma and patients who have received a maximum of two courses of melphalan, prednisone or VMCP may be included in the study.
- The patient must give informed consent according to the rules of the hospital.
- Inclusion Criteria for IFN maintenance and PBSCT or ABMT:
- At least PR after induction.
- WHO performance status 0-2.
- Suitable peripheral stem of bone marrow graft.
- No active infections.
- Absence of severe cardiac, pulmonary, neurologic or psychiatric disease.
- Serum creatinine, bilirubine and transaminases of less than 2.5 times the upper limit of normal values.
- Platelet count > 50 x 109/l.
- Absolute neutrophil count > 1 x 109/l.
- Informed consent.
- Exclusion Criteria:
- Age above 65 years.
- WHO performance status 4.
- Severe cardiac, pulmonary, neurologic or metabolis disease.
- Inadequate liver function.
- Patients with prior malignancies, except non-melanoma skin tumors or stage 0 cervical carcinoma.
- Patients with prior extensive radiotherapy involving the myelum.
Registration Details
Registration
Central registration at the HOVON Data Center:
Erasmus MC Cancer Institute, Clinical Trial Center (Hs-423)
P.O. Box 2040
NL-3000 CA Rotterdam
Phone number.: +31.10.7041560 (working days 9.00-17.00)
Fax number.....: +31.10.7041028
TOP address...: http://www.hdc.hovon.nl/top
Registration criteria
The following information will be requested:
- Protocol number
- Name of caller and/or physicisan in charge.
- Institutions name.
- Patients name, date of birth and sex.
- Hospital record number.
- Stage II or III.
Participating Sites
Site
38 results
Order by
Accrual rate
Activation date
NL-Utrecht-UMCUTRECHT
54
NL-Rotterdam-ERASMUSMC
44
NL-Nijmegen-RADBOUDUMC
44
NL-Groningen-UMCG
41
NL-Rotterdam-EMCDANIEL
39
BE-Leuven-UZLEUVEN
33
NL-Enschede-MST
26
NL-Leiden-LUMC
21
NL-Amsterdam-AMC
17
NL-Nieuwegein-ANTONIUS
16
NL-Den Haag-HAGA
13
NL-Amersfoort-MEANDERMC
12
NL-Leeuwarden-MCL
9
NL-Maastricht-MUMC
8
NL-Zwolle-ISALA
7
NL-Rotterdam-SFG
6
NL-Sittard-Geleen-ZUYDERLAND
6
NL-Amsterdam-AVL
5
NL-Rotterdam-MAASSTADZIEKENHUIS
5
NL-Hilversum-TERGOOI
4
NL-Den Bosch-JBZ
4
NL-Heerlen-ATRIUMMC
4
NL-Amsterdam-OLVG
4
NL-Haarlem-SPAARNEHAARLEM
4
NL-Geldrop-STANNA
3
NL-Amsterdam-SLOTERVAART
3
NL-Schiedam-FRANCISCUSVLIETLAND
3
NL-Purmerend-DIJKLANDERPURMEREND
3
NL-Leiderdorp-ALRIJNELEIDERDORP
2
NL-Roosendaal-BRAVIS
2
NL-Spijkenisse-SPIJKENISSEMC
1
NL-Terneuzen-ZORGSAAM
1
NL-Rotterdam-IKAZIA
1
NL-Dordrecht-ASZ
1
NL-Utrecht-DIAKONESSENUTRECHT
1
NL-Gouda-GROENEHART
1
NL-Breda-AMPHIA
1
NL-Woerden-ANTONIUSWOERDEN
1
