HOVON HO27 NHL
Archived
Main info
- Identifier:
- HOVON 27 NHL 94
- Sponsor:
- HOVON
- Included patients:
-
98
- Active sites:
-
0(of 12)12 sites are pending
- Title:
Triple High-Dose Chemotherapy with Autologous Stem Cell Support as First Line Treatment for High-Risk Non Hodgkin?s Lymphoma: A Phase II Study.
Timeline
Scheduled
Actual
1996
15 Jan
Activated
2006
15 Jan
Archived
Flow
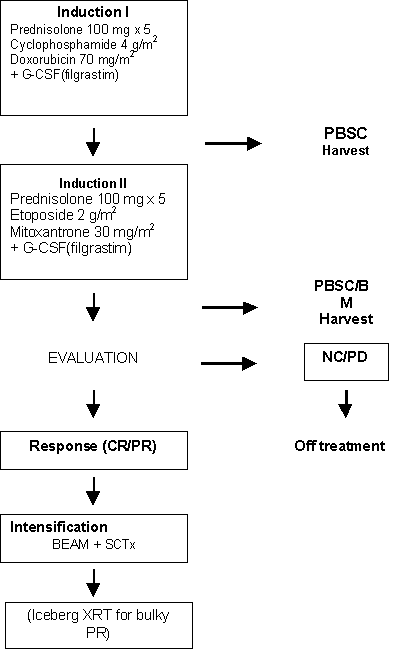
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Biopsy proven NHL of high or intermediate histology according to the Working Formulation.
- No prior treatment.
- Belonging to the high risk group.
- Ages between 15 and 65 years.
- Exclusion Criteria:
- HIV positivity.
- Prior malignancy, except skin (non-melanoma) or cervix carcinoma stage I.
- Severe major organ dysfunction or metabolic disease.
- Inadequate liver or renal function, unless due to NHL.
- WHO performance > 2.
- Inability to give informed consent.
- CNS involvement of NHL.
- Acute lymphoblastic leukemia.
- WF groups I,J, stage I non-bulky and LDH < 1.5 x normal.
Participating Sites
Site
12 results
Order by
Accrual rate
Activation date
NL-Nijmegen-RADBOUDUMC
17
NL-Utrecht-UMCUTRECHT
16
NL-Amsterdam-VUMC
12
NL-Groningen-UMCG
10
NL-Den Haag-HAGA
10
NL-Rotterdam-EMCDANIEL
9
NL-Rotterdam-ERASMUSMC
7
NL-Maastricht-MUMC
5
NL-Amsterdam-AMC
4
NL-Amersfoort-MEANDERMC
4
NL-Zwolle-ISALA
3
NL-Enschede-MST
1
