HOVON HO30 AML
Archived
Main info
- Identifier:
- HO30 AML
- Sponsor:
- HOVON
- Included patients:
-
82
- Active sites:
-
18(of 18)
- Title:
Addition of Cyclosporin A to the Combination of Mitoxantrone and Etoposide to Overcome Resistance to Chemotherapy in Refractory Acute Myeloid Leukemia: A Randomized Phase II Study.
Timeline
Scheduled
Actual
1995
15 Jan
Activated
2005
15 Jan
Archived
Flow
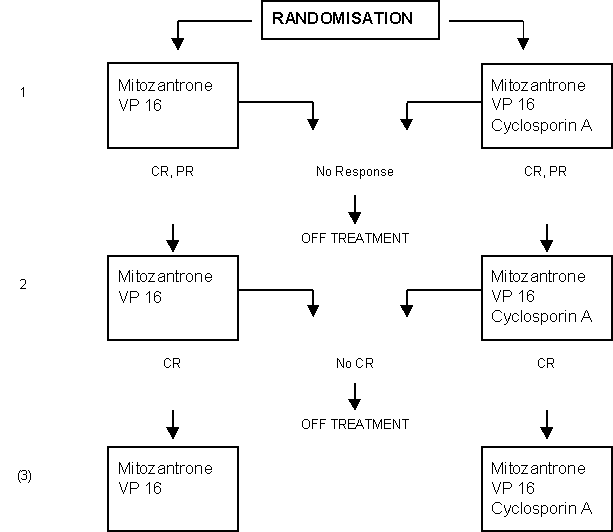
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Newly diagnosed adult AML patients in sufficiently good condition who did not attain CR after standardized chemotherapy courses with idarubicin plus Ara-C and AMSA plus Ara-C respectively (HOVON 29).
- Adult patients with AML in first or subsequent relapse after a previously attained CR.
- Exclusion Criteria:
- AML patients not fulfilling the above inclusion criteria.
- Patients with myelodysplasia.
- Severe cardiac, pulmonary, neurologic or metabolic disease.
- Suboptimal kidney function.
- Uncontrolled hypertension.
- Liver function abnormalities.
- Severe infection not controllable with adequate antimicrobial therapy.
- Serological positivity for HIV.
- Concomitant malignancy except non-melanomatous skin tumors and carcinoma in situ of the cervix uteri.
- Pregnancy.
- Known intolerance for one of the study drugs.
- Refusal of the patient.
Participating Sites
Site
18 results
Order by
Accrual rate
Activation date
NL-Groningen-UMCG
20
BE-Leuven-UZLEUVEN
14
NL-Rotterdam-EMCDANIEL
7
NL-Rotterdam-ERASMUSMC
7
NL-Den Haag-HAGA
7
NL-Amsterdam-VUMC
5
NL-Zwolle-ISALA
5
NL-Maastricht-MUMC
4
NL-Amersfoort-MEANDERMC
4
NL-Utrecht-UMCUTRECHT
4
CH-Basel-USB
3
BE-Bruxelles-STLUC
2
NL-Amsterdam-AMC
NL-Hilversum-TERGOOI
NL-Nieuwegein-ANTONIUS
CH-Bern-INSEL
CH-Zürich-USZ
CH-St. Gallen-KSSG
