HOVON HO37 ALL
Archived
Main info
- Identifier:
- HO37 ALL
- Sponsor:
- HOVON
- Included patients:
-
249
- Active sites:
-
14(of 14)
- Title:
Early Intensification by (un)related Allogenic or Autologous Stem Cell Transplantation in Adult Acute Lymphoblastic Leukemia: A Phase II Study.
Timeline
Scheduled
Actual
1999
15 Apr
Activated
2005
25 Nov
ClosedForInclusionActualStart
2010
27 Apr
CloseoutInProgressLastPtOutActualStart
2015
25 Nov
Archived
2025
27 Apr
Destruction
Flow
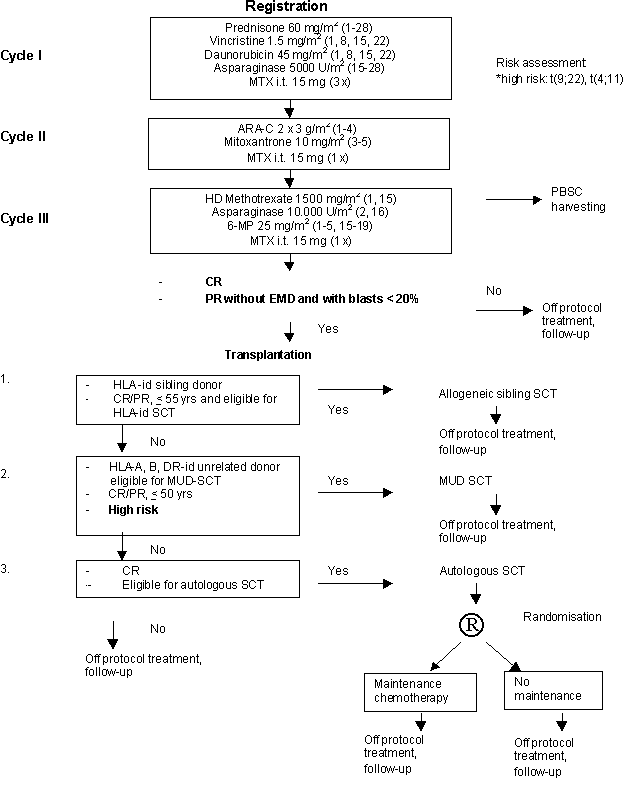
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Age between 16 and 59 (inclusive) years.
- Previously untreated with chemotherapy.
- ALL according to the FAB criteria and immunological marker analysis (B-precursor ALL, T-ALL and AUL)
- WHO performance status grade 0, 1, 2 or 3.
- Patient informed consent.
Patients will be randomised for maintenance chemotherapy one week prior to the start of the conditioning regimen of autologous SCT, after informed consent has been obtained for either no therapy or maintenance therapy for one year.
Criteria for Start of Maintenance Therapy
- CR after autologous SCT.
- Hematological recovery.
a. leukocytes > 3 x 109/l.
b. platelets > 80 x 109/l.- WHO performance status 0, 1, 2.
- Exclusion Criteria:
- B-ALL
- Severe cardiac, pulmonary, hepatic, renal, neurologic, psychiatric or metabolic disease.
- Second malignant disease, except cervix carcinoma stage 1 and non-melanoma skin cancer.
- Persisting renal insufficiency.
- Active uncontrolled infections.
- HIV positivity.
Exclusion Criteria for Start of Maintenance Therapy
- Severe toxicity CTC grade > 1.
- No BM-recovery after 3 months.
- Previous allergic lung reaction to MTX.
Participating Sites
Site
14 results
Order by
Accrual rate
Activation date
BE-Leuven-UZLEUVEN
40
NL-Amsterdam-VUMC
30
NL-Utrecht-UMCUTRECHT
28
NL-Rotterdam-EMCDANIEL
24
NL-Maastricht-MUMC
22
NL-Zwolle-ISALA
18
NL-Rotterdam-ERASMUSMC
16
NL-Den Haag-HAGA
13
NL-Nieuwegein-ANTONIUS
8
NL-Amersfoort-MEANDERMC
7
NL-Enschede-MST
6
NL-Heerlen-ATRIUMMC
2
NL-Amsterdam-AVL
1
NL-Schiedam-FRANCISCUSVLIETLAND
