HOVON HO38
Archived
Main info
- Identifier:
- HO38 CML
- Sponsor:
- HOVON
- Included patients:
-
102
- Active sites:
-
22(of 22)
- Title:
High-dose Cytarabin versus low-dose Cytarabin plus Interferon-Alpha-2a both followed by maintenance with Interferon-Alpha-2a in Chronic Myeloid Leukemia: A Phase III study by HOVON and the Belgian CMC Study Group.
Timeline
Scheduled
Actual
1998
15 Jan
Activated
1998
15 Jan
EC Approval
2001
15 Jun
ClosedForInclusionActualStart
2011
15 Jun
Archived
2026
15 Jun
Destruction
Flow
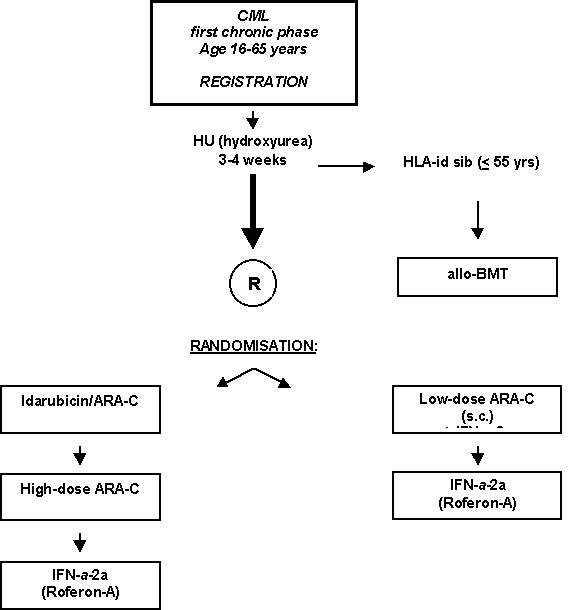
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Newly diagnosed patients with CML in first phase < 6 months.
- Presence of Philadelphia chromosome or BCL/ABL rearrangement.
- Age: 16-65 years inclusive.
- WHO performance scale 0, 1, 2.
- Informed consent.
- Exclusion Criteria:
- CML in blastic phase.
- CML in accelerated phase.
- CML without Ph chromosome or BCL/ABL rearrangement.
- Hepatic dysfunction.
- Renal dysfunction.
- Patients with severe cardiac, pulmonary or neurologic disease.
- Pregnant or lactating females.
- Human Immunodeficiency Virus (HIV) infection.
- Other malignancies, except stage I cervix carcinoma and basocellular carcinoma.
Participating Sites
Site
22 results
Order by
Accrual rate
Activation date
NL-Amsterdam-VUMC
16
NL-Utrecht-UMCUTRECHT
15
NL-Rotterdam-EMCDANIEL
12
BE-Leuven-UZLEUVEN
11
NL-Groningen-UMCG
10
NL-Rotterdam-ERASMUSMC
8
NL-Enschede-MST
4
NL-Maastricht-MUMC
4
BE-Antwerpen-ZNASTUIVENBERG
4
NL-Amersfoort-MEANDERMC
4
NL-Zwolle-ISALA
3
NL-Heerlen-ATRIUMMC
2
NL-Den Haag-HAGA
2
NL-Roosendaal-BRAVIS
2
BE-Bruxelles-STLUC
1
NL-Nijmegen-RADBOUDUMC
1
NL-Nieuwegein-ANTONIUS
1
NL-Den Bosch-JBZ
1
NL-Apeldoorn-GELREAPELDOORN
1
BE-Antwerpen-ZNAMIDDELHEIM
NL-Amsterdam-AMC
NL-Alkmaar-NWZ
