HOVON HO39
Archived
Main info
- Identifier:
- HOVON 39 NHL / EORTC 20981
- Sponsor:
- HOVON
- Included patients:
-
465
- Active sites:
-
31(of 33)1 sites are pending
- Title:
Chimeric Anti-CD20 Monoclonal Antibody (Mabthera) in Remission Induction and Maintenance Treatment of Relapsed Follicular Non-Hodgkin's Lymphoma: A Phase III Randomised Clinical Trial & Intergroup Collaborative Study (EORTC 20981).
Timeline
Scheduled
Actual
1998
01 Nov
Activated
1998
01 Nov
FPI
2009
19 Jan
Archived
2017
19 Jul
CloseoutInProgressLastPtOutActualStart
2032
19 Jul
Destruction
Flow
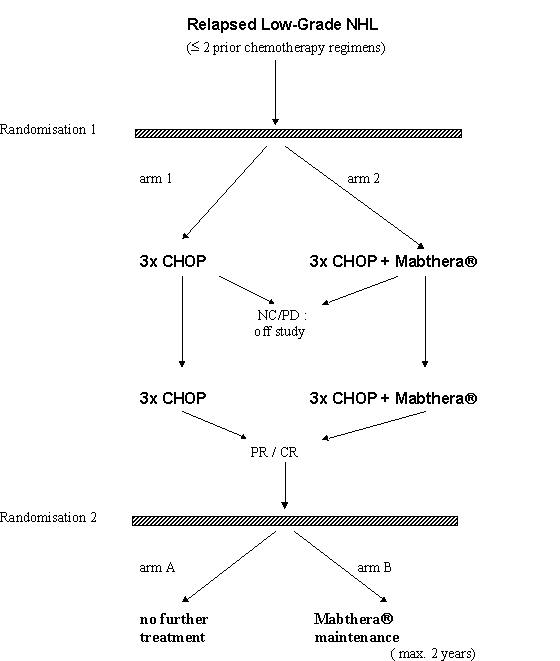
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Follicular NHL according to the REAL classification.
- Patients with Ann Arbor stages III or IV follicular NHL who have relapsed after a maximum of two adequate non-anthracycline containing systemic chemotherapy regimens.
- Patients with either remission (CR, PR), no change or progression on one of a maximum of 2 prior regiments.
- To qualify for remission or NC, duration of response c.q. NC upon one of the prior regimens should have been at least 4 weeks.
- Previous treatment should have been at least 2 months of single agent therapy and / or at least 2 consecutive cycles of polychemotherapy or purine analogues.
- The lymphoma must be CD20 positive.
- At least one mass should be present measurable by 2 perpendicular diameters by either physical or radiological examination.
- Age : 18 years or older.
- WHO Performance Status 0, 1 or 2.
- Patient information and written informed consent according to the rules of the respective country c.q institute.
Eligibility criteria for second randomisation:
- Patients in complete or partial remission at least 4 weeks after the last of 6 cycles of CHOP Mabthera.
- Patients in complete or partial remission at least 4 weeks after the last of at least 3 cycles of CHOP Mabthera, if this treatment had to be stopped because of CHOP-related adverse event.
- For patients treated with CHOP + Mabthera during remission induction: no Mabthera-related adverse event necessitating stopping of Mabthera administration.
- Time interval since last cycle of CHOP Mabthera between 4 and 8 weeks.
- IgG levels >= 3 gr/l.
- No active infection.
- Written informed consent.
- Exclusion Criteria:
- Prior treatment with more than two adequate regimens of systemic chemotherapy, or prior treatment with inadequate systemic chemotherapy.
- Prior treatment with anthracyclines / Mitoxantrone.
- Prior treatment with Mabthera.
- Circulating tumor cells > 10x109/l.
- Low grade lymphoma transformed into intermediate / high grade lymphoma.
- Patients with severe cardiac, pulmonary, neurologic, psychiatric or metabolic disease.
- Serum creatinine, BUN, alkaline phosphatase or bilirubin: > 2.5 times the upper limit of the normal value, unless clearly related to NHL.
- Pregnant women.
- Patients with prior malignancies except non-melanoma skin tumors, stage 0 cervical carcinoma or curative surgical therapy longer than 5 years ago.
- Patients with known HIV positivity.
- Patients with symptomatic CNS lymphoma.
- Patients with bone marrow involvement only.
- Patients with uncontrolled asthma or allergy, requiring steroid treatment.
- Patients who are unable to attend regular outpatient appointments for treatment and treatment evaluation.
- Patients with IgG levels < 3 gr/l.
- Prior allogenic or autologous stem cell transplantation.
- Patients considered in near future for peripheral blood stem cell collection using chemotherapy for mobilisation.
- Patients known to have any hypersensitivity or anaphylactic reactions to murine proteins or to any component of this product.
Participating Sites
Site
32 results
Order by
Accrual rate
Activation date
Non-HOVON-Sites
388
NL-Rotterdam-EMCDANIEL
22
NL-Amsterdam-AVL
7
NL-Rotterdam-ERASMUSMC
6
NL-Amsterdam-VUMC
6
NL-Groningen-UMCG
4
BE-Leuven-UZLEUVEN
4
NL-Rotterdam-MAASSTADZIEKENHUIS
3
NL-Hengelo-ZGTHENGELO
3
NL-Enschede-MST
3
NL-Amersfoort-MEANDERMC
3
NL-Dordrecht-ASZ
2
NL-Roosendaal-BRAVIS
2
NL-Hoorn-DIJKLANDERHOORN
2
NL-Tilburg-TWEESTEDEN
2
NL-Roermond-LZR
1
NL-Delft-RDGG
1
NL-Den Haag-HAGA
1
NL-Dirksland-VANWEELBETHESDA
1
NL-Heerlen-ATRIUMMC
1
NL-Hilversum-TERGOOI
1
NL-Schiedam-FRANCISCUSVLIETLAND
NL-Capelle a/d IJssel-YSL
NL-Vlaardingen-VLIETLANDVLARDINGEN
NL-Utrecht-UMCUTRECHT
NL-Nieuwegein-ANTONIUS
NL-Maastricht-MUMC
NL-Leidschendam-HMCANTONIUSHOVE
NL-Den Bosch-JBZ
NL-Amsterdam-SLOTERVAART
NL-Amsterdam-AMC
NL-Hoogeveen-TRENTBETHESDA
