HOVON HO40
Main info
- Identifier:
- HOVON 40 NHL
- Sponsor:
- HOVON
- Included patients:
-
97
- Active sites:
-
18(of 19)
- Title:
Intensified CHOP followed by Triple High-Dose Chemotherapy with Autologous Stem Cell Support as First Line Treatment for High-Risk Non Hodgkin Lymphoma: A Phase II Study.
Timeline
News
RTF-CCR SAKK clinical research grant - open for application
CALL FOR RESEARCH PROPOSAL
Rising Tide Foundation for Clinical Cancer Research (RTF-CCR) is proud to enter into its third year of Clinical Cancer Research Grant partnership with the Swiss Group for Clinical Cancer Research (SAKK) and Gateway for Cancer Research (Gateway).
Application deadline: May 20, 2015. Click here for more information and application downloads.
The grant is focused on addressing 5 critical challenges in clinical cancer research and endowed with a total of USD 1.500.000. Investigators are challenged to submit research proposals for innovative, patient-centered clinical cancer trials.
The winning proposal will be announced in November 2015.
Flow
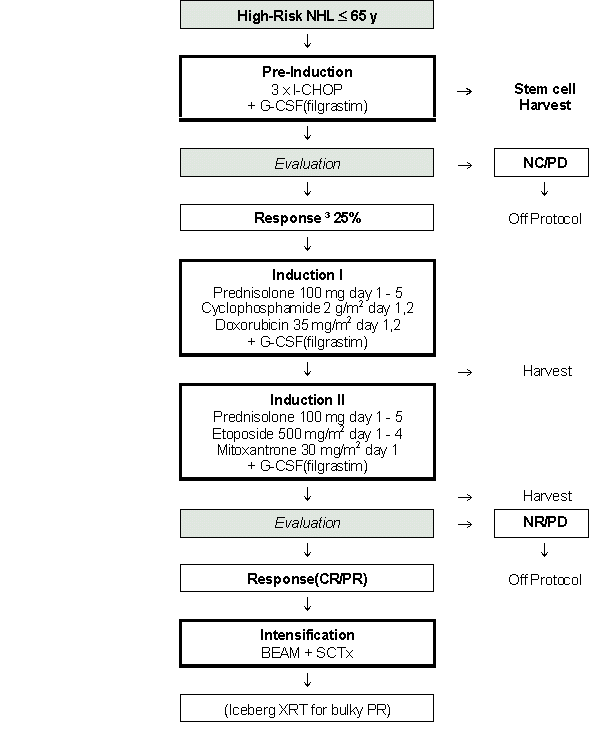
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Biopsy proven primary NHL of high or intermediate histology.
- No prior treatment
- Belonging to the high risk group
- Measurable or evaluable tumour lesion
- WHO performance 0, 1 or 2
- Ages between 18 and 65 years
- Informed consent
- Exclusion Criteria:
- Mantle cell lymphoma
- Lymphoblastic lymphoma
- Burkitt?s lymphoma
- HIV positivity
- Prior malignancy, except skin (non-melanoma), or cervix carcinoma stage I
- Severe major organ dysfunction or metabolic disease
- Inadequate liver or renal function, unless due to NHL
- CNS involvement of NHL
