HOVON HO42 AML
Main info
- Identifier:
- HO42 AML
- Sponsor:
- HOVON
- Working group party:
- Leukemia
- Age:
- 18-60
- Stage:
- 1st Line
- Echelon:
- Limited Site Selection
- Included patients:
-
1018(of 800)
- Active sites:
-
37(of 38)
- Title:
Randomized induction and post induction therapy in adult patients (<= 60 yrs of age) with acute myelocytic leukemia (AML) or refractory anemia with excess of blasts (RAEB, RAEB-t) with IPSS score >= 1.5
Timeline
News
The HO42 AML study has been published in the New England Journal of Medicine. This resulted in a lot of media attention, please find a selection of the publications here.
Flow
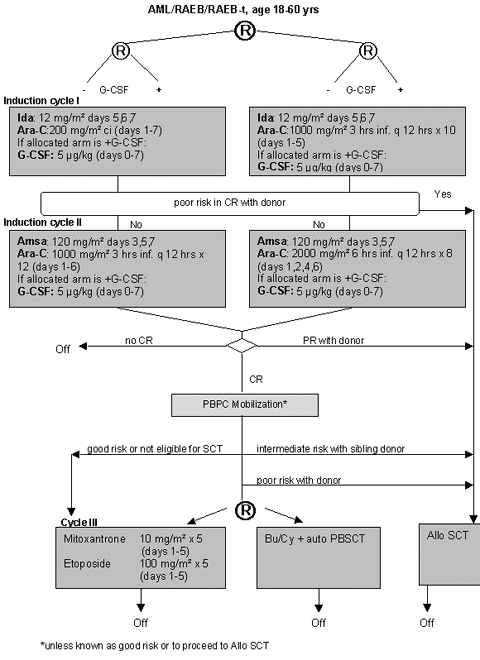
Details
- Phase:
- Select:
- Monitoring Type:
- Not any more
- Objectives:
- To study in a randomized comparison the use of granulocyte-colony stimulating factor (GCSF) for priming during induction cycles I and II as regards the complete remission rate, disease free survival, risk of relapse and overall survival.
- To evaluate the outcome of allogeneic sibling SCT in intermediate risk patients and compare the results with those after chemotherapy and ablation + PBSCT
- To assess the value of early allogeneic family donor and unrelated donor SCT in patients with poor risk AML in comparison to the results in those treated with chemotherapy and ablation + PBSCT
- To determine the prognostic value of molecular markers and gene expression profiles of the leukemia assessed at diagnosis
- To assess minimal residual disease following therapy by standardized sampling of marrow/blood
Eligibility
- Inclusion Criteria:
- Age 18-60 years (incl.)
- Subjects with a cytopathologically confirmed diagnosis of
(a) AML (M0-M2 and M4-M7, FAB classification, appendix A), or
(b) with refractory anemia with excess of blasts (RAEB) or refractory anemia with excess of blasts in transformation (RAEB-t) with an IPSS score of >1.5 (appendix B)- Patients with therapy-related AML/RAEB/RAEB-t are eligible provided they have not received chemotherapy during the past 6 months. Also patients with biphenotypic leukemia may be included.
- Subjects with a secondary AML progressing from antecedent myelodysplasia are eligible. Antecedent MDS refers to a condition of at least 4 month duration
- WHO performance status ≤ 2 (see appendix E)
- Written informed consent
- Exclusion Criteria:
- Prior chemotherapy within 6 months of study entry
- Relapse of AML or MDS after induction chemotherapy
- Prior stem cell transplant
- Previous polycythemia rubra vera
- Primary myelofibrosis
- Blast crisis of chronic myeloid leukemia
- AML-FAB type M3 or AML with cytogenetic abnormality t(15;17) or AML with a PML/RAR alpha or a variant RAR alpha fusion gene
- Impaired hepatic or renal function as defined by:
- ALT and/or AST > 3 x normal value
- Bilirubin > 3 x normal value
- Serum creatinin > 3 x normal value (after adequate hydration), (unless these are most likely caused by AML organ infiltration)
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, infection, hypertension, etc.)
- Cardiac dysfunction as defined by:
- Myocardial infarction within the last 6 months of study entry, or
- Reduced left ventricular function with an ejection fraction <50% as measured by MUGA scan or echocardiogram (another method for measuring cardiac function is acceptable)
- Unstable angina
- Unstable cardiac arrhythmias
- Pregnancy
Registration Details
Eligible patients should be registered immediately after diagnosis (on the basis of cytological examination of marrow and blood smears in the participating center), and before the start of chemotherapy. Patients can be registered at the HOVON Data Center of the Erasmus MC Daniel den Hoed by phone call: +31.10.4391568 or fax +31.10.4391028 Monday through Friday, from 09:00 to 17:00, or via the Internet through TOP (Trial Online Process; https://www.hdc.hovon.nl/top). A logon to TOP can be requested at the HOVON Data Center for participants.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Patients initials or code
- Patients hospital record number
- Sex
- Date of birth
- Date of diagnosis of AML or RAEB or RAEB-t
- WHO performance status
- White blood cell count (WBC)
- FAB type of AML or RAEB or RAEB-t
- Eligibility criteria (see 8.1.1 and 8.1.2)
- Prior hematological or oncological disease
- Previous chemotherapy or radiotherapy
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
