HOVON HO45 NHL
Archived
Main info
- Identifier:
- HOVON 45 MCL
- Sponsor:
- HOVON
- Included patients:
-
88
- Active sites:
-
16(of 17)
- Title:
High dose chemotherapy with cytoreduction and in vivo purging of the peripheral stem cell transplant by anti-CD20 (MabThera) in the treatment of mantle cell lymphoma.
Timeline
Scheduled
Actual
2001
15 Mar
Activated
2004
15 Nov
ClosedForInclusionActualStart
2015
13 Oct
Archived
2030
13 Oct
Destruction
Flow
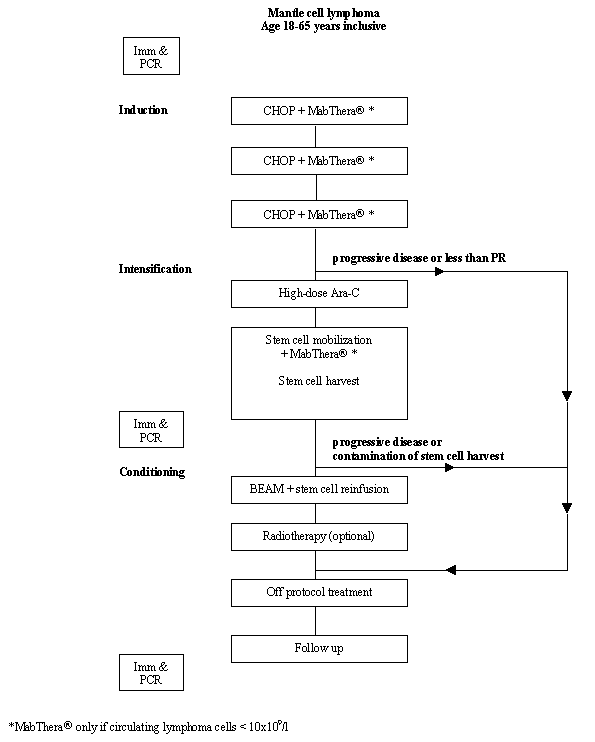
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
Eligibility
- Inclusion Criteria:
- Patients with histologically and immunologically proven mantle cell lymphoma (CD19+, CD5+, CD23-, Ig-light chain monoclonal, Cyclin-D1protein+)
- Ann Arbor stage II - IV
- CD20 positive
- Age 18-65 years inclusive
- WHO performance status 0 - 2
- Written informed consent
- Exclusion Criteria:
- Prior treatment
- Severe organ dysfunction not due to the lymphoma
- Bilirubin > 2.5 x normal
- Creatinine > 150 ��mol/l (not due to lymphoma)
- History of active cancer during the past 5 years, except basal carcinoma of the skin or carcinoma in situ of the cervix
- Uncontrolled infection
- Central nervous system involvement of lymphoma
- Pregnancy
- HIV positivity
Participating Sites
Site
16 results
Order by
Accrual rate
Activation date
NL-Rotterdam-ERASMUSMC
17
NL-Nijmegen-RADBOUDUMC
15
NL-Groningen-UMCG
13
NL-Amsterdam-AMC
12
NL-Amsterdam-VUMC
10
NL-Den Haag-HAGA
7
NL-Zwolle-ISALA
4
NL-Rotterdam-EMCDANIEL
4
NL-Nieuwegein-ANTONIUS
1
NL-Enschede-MST
1
NL-Utrecht-DIAKONESSENUTRECHT
1
NL-Utrecht-UMCUTRECHT
1
NL-Amersfoort-MEANDERMC
1
NL-Leiden-LUMC
1
NL-Roosendaal-BRAVIS
NL-Deventer-DZ
