HOVON HO46
Archived
Main info
- Identifier:
- HOVON 46 NHL
- Sponsor:
- HOVON
- Included patients:
-
268
- Active sites:
-
0(of 51)51 sites are pending
- Title:
A randomized phase III study of chimeric anti-CD20 monoclonal antibody (Rituximab) with 2-weekly CHOP chemotherapy in elderly patients with intermediate- or high-risk non-Hodgkin?s lymphoma.
Timeline
Scheduled
Actual
2001
28 Nov
Activated
2005
06 Sep
CloseoutInProgressLastPtOutActualStart
2015
13 Oct
Archived
2030
13 Oct
Destruction
Flow
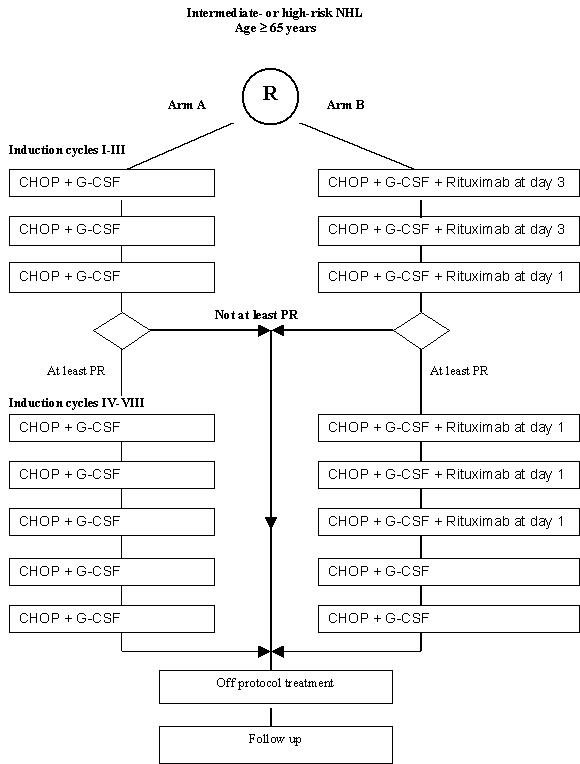
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Patients with a confirmed histologic diagnosis of NHL according to the WHO classification (Appendix A):
a. Mantle cell lymphoma (MCL)
b. Follicular lymphoma (grade III) (FL III)
c. Diffuse large B-cell lymphoma (DLBCL)- Low-intermediate, high-intermediate or high risk NHL according to age-adjusted IPI score (appendix G)
- NHL must be CD20 positive.
- Age >= 65 years
- WHO performance status <= 2 (see appendix E)
- Written informed consent.
- Exclusion Criteria:
- Intolerance of exogenous protein administration
Severe cardiac dysfunction (NYHA classification II-IV, appendix F) or LVEF < 45 %
- Significant renal dysfunction (serum creatinin >= 150 umol/l), unless related to NHL
- Significant hepatic dysfunction (total bilirubin >= 30 umol/l or transaminases >= 2.5 times normal level), unless related to NHL
- Suspected or documented Central Nervous System involvement by NHL
- Patients known to be HIV-positive
- Patients with active, uncontrolled infections
- Patients with uncontrolled asthma or allergy, requiring steroid treatment
- Prior treatment with chemotherapy, radiotherapy or immunotherapy for this lymphoma, except local radiotherapy in case of (potential) organ dysfunction by localized lymphoma mass or infiltration
- History of active cancer during the past 5 years, except basal carcinoma of the skin or stage 0 cervical carcinoma
Participating Sites
Site
51 results
Order by
Accrual rate
Activation date
NL-Nieuwegein-ANTONIUS
19
NL-Rotterdam-ERASMUSMC
18
NL-Amsterdam-VUMC
17
NO-Oslo-RADIUM
15
SE-Lund-SUH
12
NL-Zwolle-ISALA
11
NL-Amersfoort-MEANDERMC
10
NL-Amsterdam-AMC
9
NL-Den Haag-HAGA
9
NL-Enschede-MST
8
NL-Rotterdam-EMCDANIEL
8
SE-Sundsvall-RVN
7
NL-Rotterdam-MAASSTADZIEKENHUIS
7
NL-Utrecht-DIAKONESSENUTRECHT
6
NL-Delft-RDGG
6
NL-Meppel-ISALADIACONESSEN
5
NL-Dordrecht-ASZ
5
NL-Leidschendam-HMCANTONIUSHOVE
5
SE-Halmstad-HALLANDS
5
NO-Stavanger-HELSESTAVANGER
5
BE-Leuven-UZLEUVEN
4
SE-Luleå-SUNDERBY
4
SE-Goteborg-SAHLGRENSKA
3
NL-Den Haag-HMCWESTEINDE
3
NL-Delfzijl-OMMELANDERDELFZIJL
3
NL-Stadskanaal-TRENTREFAJA
3
NL-Apeldoorn-GELREAPELDOORN
3
SE-Uppsala-UPPSALAUH
3
NL-Amsterdam-AVL
3
NL-Hengelo-ZGTHENGELO
3
SE-Malmö-UMAS
3
NL-Den Bosch-JBZ
2
FI-Turku-TYKS
2
NL-Breda-AMPHIA
2
NL-Groningen-UMCG
2
NL-Utrecht-UMCUTRECHT
2
NL-Emmen-SCHEPER
2
NL-Venlo-VIECURI
2
NL-Haarlem-SPAARNEHAARLEM
2
NL-Helmond-ELKERLIEK
2
NL-Groningen-MARTINI
2
NL-Capelle a/d IJssel-YSL
2
NL-Roosendaal-BRAVIS
1
NO-Bergen-HELSEBERGEN
1
NL-Amsterdam-SLOTERVAART
1
NO-Tromsø-NORTHNOORWEGEN
1
SE-Linköping-REGIONOSTERGOTLAND
1
NL-Leiden-ALRIJNLEIDEN
1
NL-Goes-ADRZ
1
NL-Vlaardingen-VLIETLANDVLARDINGEN
1
SE-Stockholm-KAROLINSKASOLNA
