HOVON HO47
Archived
Main info
- Identifier:
- HOVON 47 NHL
- Sponsor:
- HOVON
- Included patients:
-
15
- Active sites:
-
13(of 13)
- Title:
Chlorambucil versus 2 x 2 Gy involved field radiotherapy in stage III/IV previously untreated follicular lymphoma patients. A prospective, randomized phase III clinical trial.
Timeline
Scheduled
Actual
2001
04 Oct
Activated
2014
04 Oct
Archived
Flow
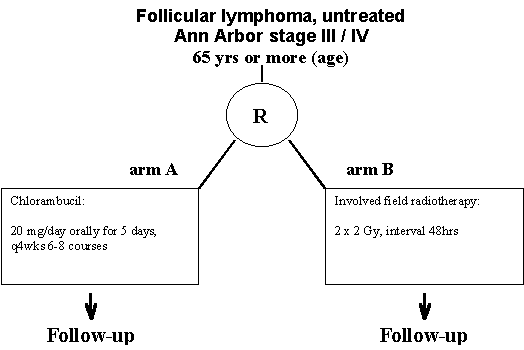
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Previously untreated Ann Arbor stage III and IV follicular lymphoma patients, grade I � III. (see appendices A and C)
- Age 65 years or more, no upper limit.
- WHO Performance Status 0 - 2. (see appendix E)
- Nodal and extranodal sites, measurable bidimensionally on physical examination or diagnostic imaging.
- Adequate haematological parameters at diagnosis: Hb >= 6.0 mmol/l, leukocytes >= 4.0 x 109 /l, thrombocytes >= 100 x 109 /l.
- Written informed consent.
- Exclusion Criteria:
- Severe cardiac, pulmonary, neurologic, psychiatric or metabolic disease precluding either Chlorambucil chemotherapy or radiotherapy.
- Patients with prior malignancies except basal cell carcinoma of the skin or carcinoma in situ of the cervix.
- Patients unable to fulfil regular outpatient appointments both for treatment and follow-up.
- Known HIV-positivity.
- Known central nervous system or orbital NHL localisation.
- Use of systemic corticosteroid medication (inhalation and topical corticosteroids are allowed).
Participating Sites
Site
13 results
Order by
Accrual rate
Activation date
NL-Breda-AMPHIA
3
NL-Amsterdam-AVL
1
NL-Roermond-LZR
1
NL-Leiden-LUMC
1
NL-Heerlen-ATRIUMMC
1
BE-Antwerpen-ZNAMIDDELHEIM
1
NL-Rotterdam-MAASSTADZIEKENHUIS
1
NL-Leidschendam-HMCANTONIUSHOVE
1
NL-Haarlem-SPAARNEHAARLEM
1
NL-Den Haag-HAGA
1
NL-Delft-RDGG
1
NL-Amsterdam-OLVG
1
NL-Tilburg-VERBEETEN
1
