Erasmus MC HO502 Other
Main info
- Identifier:
- HO502 (DUET)
- Sponsor:
- Erasmus MC
- Working group party:
- SCT & Supportive care
- Stage:
- 1st Line
- Echelon:
- Limited Site Selection
- Included patients:
-
66(of 474)
- Active sites:
-
17(of 28)8 sites are pending
- Title:
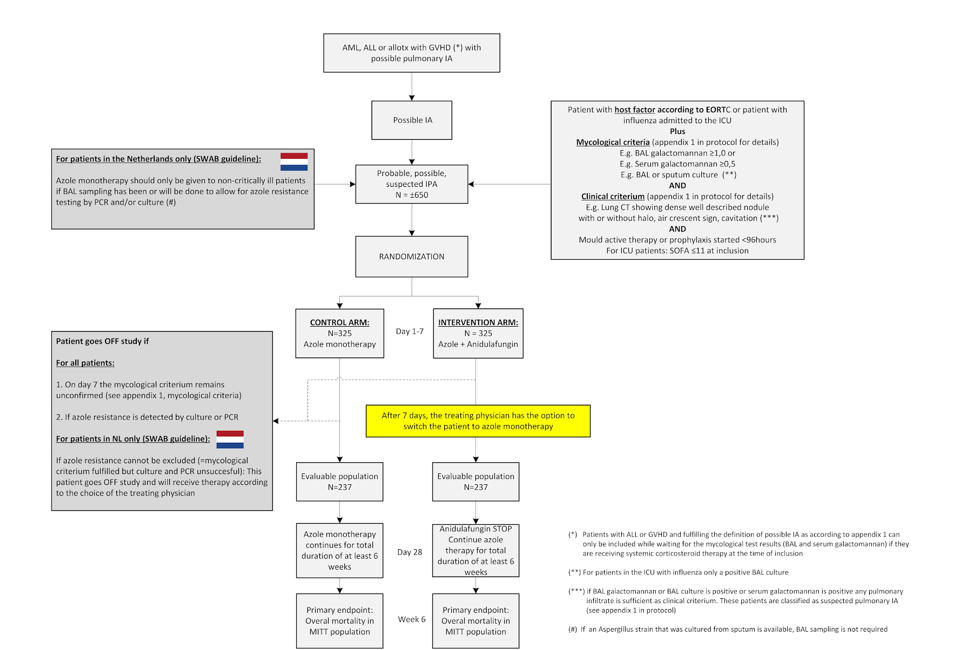
Azole-echinocandin combination therapy for invasive aspergillosis. A multicenter pragmatic randomized superiority trial
Timeline
Flow
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Site Evaluation Visit
- Objectives:
Primary objective
- Evaluate if the survival in patients with a triazole susceptible IA can be improved when the initial therapy consists of triazole and echinocandin combination therapy instead of triazole monotherapy.
Secondary objectives
- Evaluate if a triazole/echinocandin combination therapy improves the overall quality of life and if it is a cost-effective intervention
- Evaluate the outcome of patients in which a triazole-resistant A. fumigatus is detected in relation to the initial antifungal therapy they had received (i.e. triazole monotherapy or combination therapy).
- Evaluate the outcome of patients in which resistance testing is unsuccessful in function of the antifungal therapy they received.
- Evaluate if the baseline serum galactomannan value and the serum galactomannan kinetics are predictive of overall 6-week survival.
Eligibility
- Inclusion Criteria:
- 18 years or older
- Have started or will start voriconazole or isavuconazole (or posaconazole if voriconazole or isavuconazole cannot be given as per treating physician’s decision) as antifungal therapy on the baseline visit.
- For all patients: presence of one of the EORTC/MSG host factors as defined in appendix 1 (protocol) or being admitted to the ICU with influenza
- For non-ICU patients or ICU patients without influenza: Meet the EORTC/MSG clinical criterium (appendix 1, protocol)
- For non-ICU patients or ICU patients without influenza: Meet the mycological criterium (appendix 1, protocol) or fulfil inclusion criterium 7
- For ICU patients with influenza we consider an isolated positive sputum culture for Aspergillus spp. insufficient as amycological criterium. Therefore, in these patients only one of the following mycological criteria are acceptable; Serum galactomannan ≥0.5, BAL galactomannan ≥1.0 or Aspergillus spp. cultured in BAL fluid.
- Please note that patients with AML receiving chemotherapy or patients with ALL receiving or having received corticosteroid therapy within the last 4 weeks in the context of their pre-phase, induction, consolidation, intensification or interphase treatment as well as patients receiving systemic immunosuppressive therapy for GVHD can be included before the mycological criterium is fulfilled on condition that they fulfill the EORTC/MSG lung CT radiology criteria (halo sign, well-described nodule, cavity as described in appendix 1) at the time of inclusion and as long as the mycological test results are expected to become available within 96 and no later than 7 days after inclusion. If these test results turn out to be negative, the patient will be withdrawn from the study and further treatment is at physician’s discretion.
- Written informed consent by patient or legal representative.
- Exclusion Criteria:
- Known history of allergy, hypersensitivity or serious reaction to azole or echinocandin antifungals;
- Patients with chronic invasive aspergillosis or a chronic non-invasive aspergillus infection (e.g. aspergilloma) defined as the clinical or radiological sign of infection being present for >28 days.
- Receipt of itraconazole, voriconazole, posaconazole or isavuconazole as prophylaxis for at least 7 days in the 14 days preceding the date of the first radiological signs of the Aspergillus infection. Patients in which the most recent serum level of the triazole given as prophylaxis was subtherapeutic can be included (*).
- Receipt of echinocandin prophylaxis for >96 hours in the preceding 7 days
- Receipt of systemic antifungal treatment with an echinocandin or an azole for the current episode of invasive aspergillosis for a duration of > 96 hours.
- For patients in the Netherlands only: Diagnostic testing to exclude azole resistance will not be possible (sputum cultures are negative and BAL sampling will not be performed)
- ICU patients only: Patients with a sequential organ failure assessment (SOFA) score >11 at the time of screening for the study are excluded. If randomization is done >24 hours after screening the calculation should be repeated before the patient can be randomized (appendix 3, protocol)
- ICU patients only: Patients in which weaning from the ventilator or ECMO system is deemed unlikely due to irreversible lung damage
- Patients with any condition which, in the opinion of the investigator, could affect patient safety, preclude evaluation of response (e.g. because survival beyond 6 weeks is unlikely due to the underlying disease status)
- Patient previously included in this study
(*) Subtherapeutic levels are defined as itraconazole (parent compound only) <0.5 mg/L or posaconazole <0.7mg/L or voriconazole <1.0mg/L or isavuconazole <1.0mg/L
Registration Details
Eligible patients should be randomized before start of study treatment. Patients need to be registered and randomized at the HOVON Data Center by one of the following options:
- By ALEA; Use goto eCRF button > select the [Patient tab] and click the [Add new patient] button. Complete all items and click the [Submit] button
- By mailing the completed registration/randomization CRF to hdc@erasmusmc.nl
- By phone +31 (0)10 704 1560 Monday through Friday, from 09:00 to 17:00 CET
The following information will be requested at registration:
Protocol number
Institution name
Name of caller/responsible investigator
Sex
(Partial) date of birth and age at time of registration
Date written informed consent
Specific items patient gives consent for (see ICF)
Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
