HOVON HO54
Archived
Main info
- Identifier:
- HOVON 54 MM
- Sponsor:
- HOVON
- Included patients:
-
93
- Active sites:
-
6(of 6)
- Title:
Non myeloablative allogeneic stem cell transplantation following high dose therapy as part of first line therapy to induce graft versus myeloma for patients <= 65 years participating in the HOVON 50 study.
Timeline
Scheduled
Actual
2003
02 Jun
Activated
2006
07 Nov
CloseoutInProgressLastPtOutActualStart
2016
07 Nov
Archived
Flow
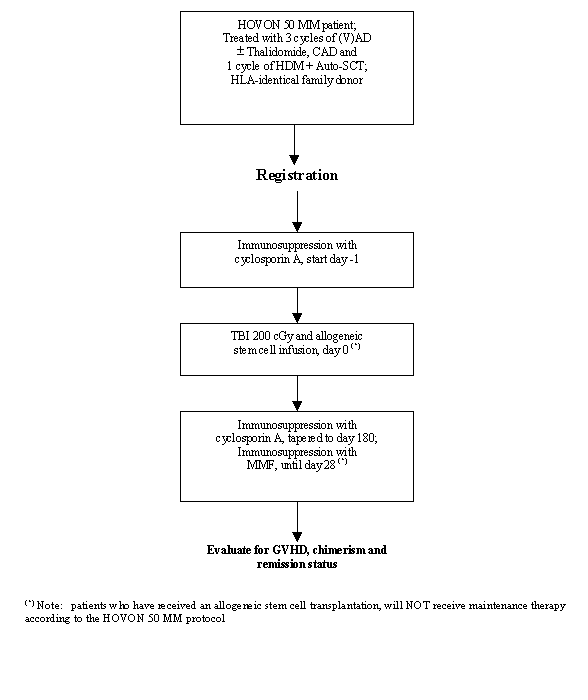
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Age 18-65 years inclusive;
- Included in the HOVON 50 MM study;
- Patient has received 3 cycles of VAD or AD+Thalidomide, CAD and 1 cycle of high dose Melphalan with autologous stem cell reinfusion according to HOVON 50 MM protocol;
- NMA allogeneic transplantation planned between 2 and 6 months after autologous stem cell reinfusion;
- WHO performance status 0-2;
- HLA-identical family donor;
- Written informed consent.
Donor Eligibility Criteria:
- HLA genotypically identical sibling;
- Informed consent to undergo G-CSF administration and leukapheresis;
- Adequate veins for leukapheresis or agrees to placement of central venous catheter;
- Willing and able to undergo apheresis procedure according to institutional guidelines.
- Exclusion Criteria:
- Creatinin clearance < 50 ml/min;
- Severe cardiac dysfunction (NYHA classification II-IV, see appendix D) ;
- Significant hepatic dysfunction (serum bilirubin >= 30 micromol/l or transaminases >= 2.5 times normal level), unless related to myeloma;
- Patients known to be HIV-positive;
- Patients with active, uncontrolled infections;
- Progressive disease / relapse from CR / progression from MR or PR after HDM with autologous stem cell reinfusion according to HOVON 50 MM protocol.
Donor exclusion criteria:
- Monozygotic identical twin;
- Age less than 12 years;
- Pregnancy;
- Known allergy to G-CSF;
- HIV positive;
- Current serious systemic illness.
Participating Sites
Site
6 results
Order by
Accrual rate
Activation date
NL-Utrecht-UMCUTRECHT
31
NL-Rotterdam-EMCDANIEL
19
NL-Nijmegen-RADBOUDUMC
19
NL-Amsterdam-VUMC
13
NL-Maastricht-MUMC
6
NL-Amsterdam-AMC
5
