HOVON HO55 NHL
Main info
- Identifier:
- HO55 NHL/MCL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- > 65
- Stage:
- 1st Line
- Included patients:
-
95(of 95)
- Active sites:
-
36(of 30)
- Title:
Efficacy of maintenance therapy with rituximab after induction chemotherapy (R-CHOP vs. R-FC) for elderly patients with mantle cell lymphoma not suitable for autologous stem cell transplantation
Timeline
News
This study has been closed on 21 October 2010.
Flow
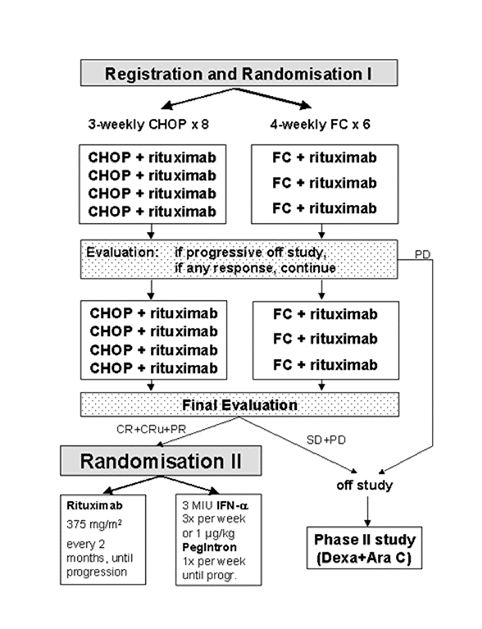
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
- To test in elderly patients with advanced mantle cell lymphoma, whether rituximab plus a combination of fludarabine with cyclophosphamide (6 FC cycles) results in a higher reduction of lymphoma mass measured by the percentage of CR than rituximab combined with the standard chemotherapy scheme (8 CHOP
cycles).
- To compare maintenance therapy with rituximab with maintenance with interferon-alpha or pegylated interferon for progression free survival, after 2 different regimens of induction chemo-immunotherapy in elderly patients with
mantle cell lymphoma.
Eligibility
- Inclusion Criteria:
- Histologically proven mantle cell lymphoma according to the WHO classification, preferably confirmed by central pathology review beforehand
- Clinical stage II, III or IV
- Previously untreated patients
- Above the age of 65 years and older or patients at the age between 60 and 65, if not eligible for high dose chemotherapy
- WHO performance ≤ 2
- Informed consent according to ICH/EU GCP and national/local regulations
- Measurable disease. If e.g. only BM infiltration, patients can only undergo a second randomization if a CR is obtained
- Exclusion Criteria:
- WHO performance of 3 or more
- Known anti-murine antibody (HAMA) reactivity or known hypersensitivity to murine antibodies
- Leukocytes <2.0x 109/l or thrombocytes <100x 109/l, unless clearly related to MCL bone marrow infiltration
- Patients previously treated for lymphoma
- Patients without measurable lesions; if e.g. only bone marrow infiltration, patients may be included, but can only undergo a second randomization in case of CR
- Patients with stage I disease
- Patients with central nervous system involvement
- Patients with a history of autoimmune hemolytic anaemia or autoimmune trombocytopenia
- Patients with serious cardiac disease (uncontrolled arrhythmias, unstable angina, severe congestive heart failure)
- Patients with serious pulmonary, neurological, endocrinological or other disorder interfering with full dosing of CHOP or FC chemotherapy
- Liver enzymes >3x normal or bilirubin >2.5x normal (not due to lymphoma)
- Creatinine >2x normal value, corrected for age and weight (not due to lymphoma)
- Patients with unresolved hepatitis B or C infection or known HIV positive infection
- Uncontrolled infection
- Patients with a serious depression that needed therapy within the last 5 years
- Any psychological, familial, sociological or geographical condition potentially hampering compliance with the study protocol and follow-up schedule
- Concomitant or previous malignancies other than basal cell or squamous cell skin cancer, in situ cervical cancer and other cancer for which the patient has been disease-free for at least 5 years
Registration Details
Registration is only accepted from authorised investigators and must be done before the start of the treatment.
Germany GCLSG: Phone: +49-89-7095-4900/01 Fax: +49-89-7095-7900/01 -
HOVON: Phone +31-10-4391568 Fax: +31-10-4391028 Internet: www.hdc.hovon.nl/top
Nordic LG: Phone +45-35-451146 Fax: +45-35-454841
France GELA
A list of questions to be answered during both randomisation procedures is included in the registration checklist, which is part of the case report forms. The checklist should be completed by the responsible investigator before the patient is randomised.
For the first randomisation the following data must be provided (see registration form):
- Institution number assigned to the responsible investigator
- Name, phone and fax number of the person requesting the randomisation
- Name of the responsible investigator
- Patient’s initials (at least 2 letters)
- Patient’s birth date (day/month/year)
- Name, address and journal-number/entry-number of the local pathology
- Eligibility criteria
- Stratification criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
