HOVON HO58
Main info
- Identifier:
- HOVON 58 ET
- Sponsor:
- HOVON
- Included patients:
-
7
- Active sites:
-
9(of 9)
- Title:
Pegylated interferon alpha-2a or anagrelide in high risk essential thrombocythemia. Observational study of low and intermediate risk patients.
Timeline
Flow
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Patients with a (previously*) confirmed diagnosis of ET according to the criteria of the WHO in appendix A
- Age 18-80 inclusive
- Written informed consent
- In case of previously diagnosed ET collagen fibrosis must be absent and reticulin fibrosis only minimal or absent in the bone marrow at entry
Registered patients are eligible for randomization if:
- they fulfill the inclusion and exclusion criteria for registration
- risk stratification is high risk
- written informed consent for randomization is obtained
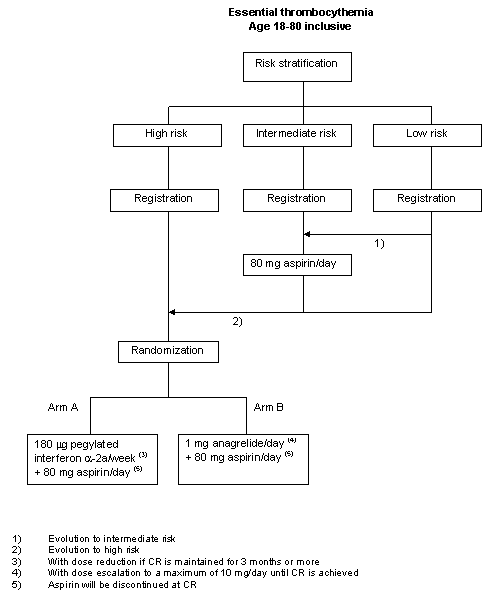
Risk stratification
Management of patients with ET will be risk based, according to risk stratification groups as defined below.Low risk - all of the following:
a. Age < 60 years
b. No history of or current bleeding, arterial, venous or microvascular thrombosis
c. Platelet count < 1500x109/l
d. No cardiovascular risk factorsHigh risk - any of the following:
a. Age >= 60 years
b. A history of or current bleeding, arterial or venous thrombosis
c. Platelet count >= 1500x109/lIntermediate risk:
a. Neither low nor high riskCardiovascular risk factors are defined as:
- Hypercholesterolaemia (total cholesterol > 6 mmol/l);
- Hypertension (systolic > 140 mmHg, diastolic > 90 mmHg);
- Diabetes mellitus (according to the WHO criteria);
Family history of cardiovascular disease (>1 first degree family member with premature (<50 years) vascular disease);
- Smoking (current smoking > 5 cigarettes per day);
- Obesitas (BMI > 30).
- Exclusion Criteria:
- Patients with renal dysfunction (creatinin > 1.5 times ULN)
- Patients with hepatic dysfunction (ALAT or ASAT > 2 times ULN or bilirubin > 1.5 times ULN)
- Patients previously treated with interferon alpha, pegylated interferon alpha-2a or anagrelide
- Patients with severe or refractory congestive cardiac failure defined as requiring multiple drug therapy
- Patients with a clinical history of class III or IV cardiac disease; defined as breathlessness or pain of cardiac origin on minimal exertion or at rest
- Patients with current severe arrhythmia e.g. ventricular tachycardia; atrial fibrillation is not an exclusion criterium
- Patients with thyroid dysfunction not responsive to therapy
- Patients who are known to be HIV-positive
- Patients with active and/or uncontrolled infection, including active hepatitis
- Patients with active malignancies
- Patients with a history of neuropsychiatric disorder requiring hospitalization
- Patients who have received any experimental therapy within 30 days prior to enrolment in this study
- Female patients who are pregnant, nursing or of reproductive potential and who are not practising effective means of contraception
