HOVON HO61
Archived
Main info
- Identifier:
- HOVON 61 NHL
- Sponsor:
- HOVON
- Active sites:
-
6(of 6)
- Title:
Supportive care in chemotherapy-related anemia in elderly non-Hodgkin's lymphoma: Darbepoetin alfa (Ara-nesp) versus red blood cell transfusion.
Timeline
Scheduled
Actual
2004
17 Nov
Activated
2020
23 Oct
Archived
Flow
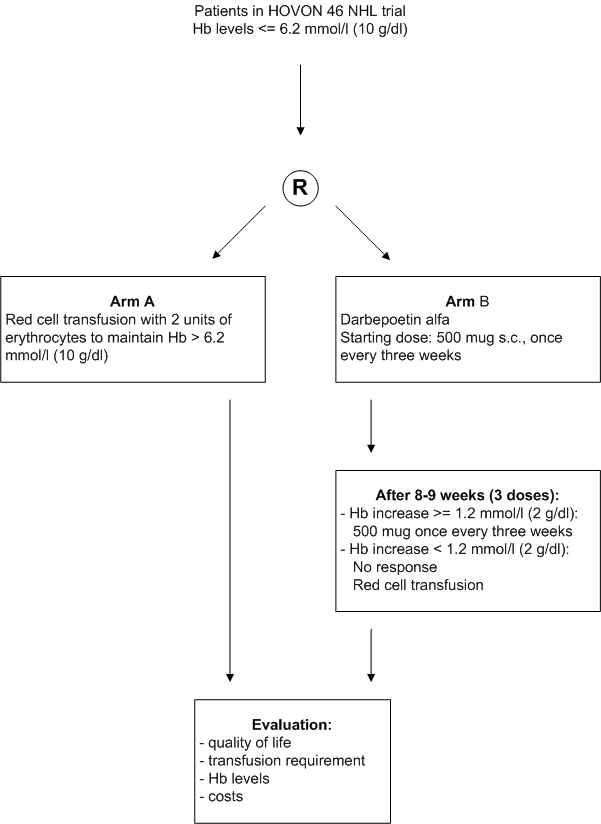
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Registration in the HOVON 46 NHL trial;
- Hb levels <= 6.2 mmol/l (<= 10 g/dl);
- Completion of baseline quality of life questionnaire;
- Written informed consent
- Exclusion Criteria:
- Hypersensitivity to components of darbepoetin;
- Treatment with epoetin or epoetin-related drugs within 4 weeks before registration in the HOVON 46 NHL trial;
- Red cell transfusion after registration in the HOVON 46 NHL trial and within 4 weeks before randomization;
- Correctable anemia not related to chemotherapy or bone-marrow infiltration such as deficiencies (iron, vit. B12, folic acid, autoimmune hemolysis);
- Anemia due to other hematological diseases such as myelodysplastic syndromes or myeloproliferative syndromes;
- Uncontrolled hypertension.
Participating Sites
Informatie is niet langer beschikbaar
Site
6 results
Order by
Accrual rate
Activation date
NL-Rotterdam-EMCDANIEL
NL-Amsterdam-VUMC
NL-Den Haag-HAGA
NL-Nieuwegein-ANTONIUS
NL-Rotterdam-ERASMUSMC
NL-Utrecht-UMCUTRECHT
