HOVON HO64
Archived
Main info
- Identifier:
- HOVON 64 ITP
- Sponsor:
- HOVON
- Included patients:
-
156
- Active sites:
-
34(of 35)
- Title:
Anti-CD20 treatment of relapsed or refractory Immune Thrombocytopenic Purpura (ITP) after first line corticosteroid treatment.
Timeline
Scheduled
Actual
2005
29 Aug
Activated
2015
13 Oct
Archived
2030
13 Oct
Destruction
Flow
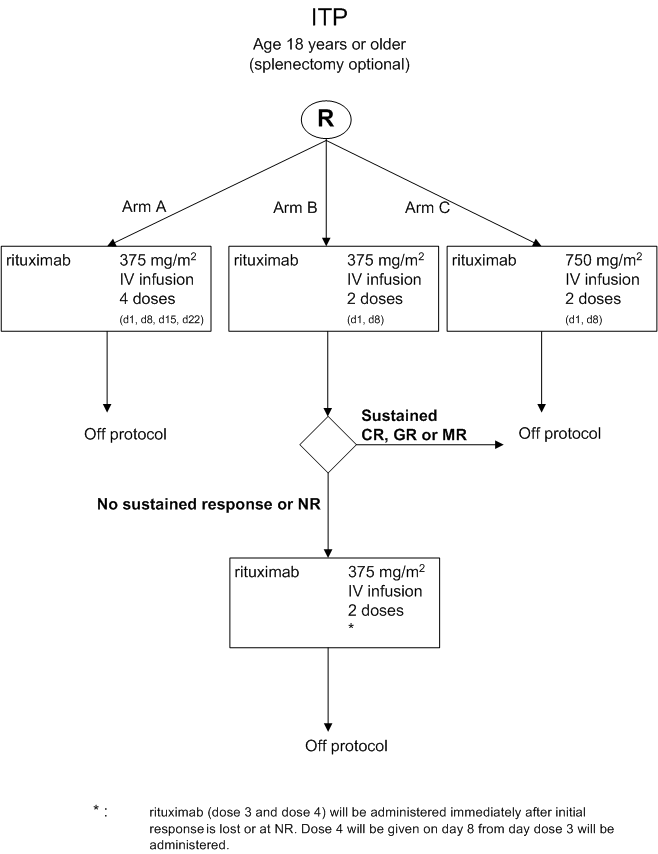
Details
- Phase:
- Prospective randomized Phase II study
- Monitoring Type:
- Objectives:
- The main objective of the current study is to investigate the effectiveness of three different dosing schedules rituximab in refractory or relapsed ITP patients, whether or not already splenectomized.
- Furthermore, stratification for splenectomy will be performed to reliably assess whether treatment of non-splenectomized patients with rituximab results in avoidance of splenectomy after 1 year follow up.
Eligibility
- Inclusion Criteria:
- Age minimal 18 years
- Subjects with relapsed or refractory ITP (fulfilling the diagnostic criteria given in appendix A) and platelet numbers <30 x 10^9/l
- Having completed first line treatment with corticosteroids
- Written informed consent
- WHO performance status <= 2
- Exclusion Criteria:
- The presence of an accessory spleen in splenectomized patients.
- Use of anticoagulants or chemotherapy or known other disorders and/or treatments influencing the platelet number within 3 months of randomization date (tranexaminic acid (Cyklokapron) treatment is allowed).
- Pulsed or high dose corticosteroids, IVIG or splenectomy within 3 weeks prior to randomization. Maintenance corticosteroid therapy is allowed.
- Prior therapy with rituximab.
- ITP treatments (other than corticosteroids, IVIG or splenectomy) within 3 months prior to randomization (e.g. cyclosporine, vincristine). Stable treatment with non-immunosuppressive medication (i.e. danazol, dapson, vitamin C) is permitted.
- Inadequate renal and liver function, i.e. creatinin or bilirubin >2.5 x the upper normal value
- Neutrophil count <1.5 x 10^9/l and hemoglobin level <6.2 mmol/l.
- Active bleeding (defined by grade 3 or 4 according to NCI CTCAE v3.0)
- Pregnant or lactating
- Systemic infections: active viral infections, including HIV
- Seriously immunocompromised patients
- Systemic autoimmune disorders (e.g. Systemic lupus erythematosus (SLE))
- Current malignant disease
- Any experimental therapy within 30 days prior to randomization.
Participating Sites
Site
34 results
Order by
Accrual rate
Activation date
NL-Rotterdam-ERASMUSMC
29
NL-Amsterdam-AMC
17
NL-Dordrecht-ASZ
12
NL-Utrecht-DIAKONESSENUTRECHT
9
NL-Leiden-LUMC
8
NL-Maastricht-MUMC
7
NL-Amsterdam-VUMC
7
NL-Den Bosch-JBZ
7
NL-Enschede-MST
6
NL-Amersfoort-MEANDERMC
6
NL-Nieuwegein-ANTONIUS
6
NL-Den Haag-HAGA
5
NL-Nijmegen-RADBOUDUMC
5
NL-Hoorn-DIJKLANDERHOORN
5
NL-Eindhoven-MAXIMAMC
5
NL-Hilversum-TERGOOI
4
NL-Hoofddorp-SPAARNEGASTHUIS
3
NL-Ede-ZGV
3
NL-Heerlen-ATRIUMMC
2
NL-Roosendaal-BRAVIS
2
NL-Purmerend-DIJKLANDERPURMEREND
2
NL-Rotterdam-MAASSTADZIEKENHUIS
1
NL-Winterswijk-SKBWINTERSWIJK
1
NL-Amsterdam-SLOTERVAART
1
NL-Amsterdam-OLVG
1
NL-Amstelveen-AMSTELLAND
NL-Lelystad-STJANSDALLELYSTAD
NL-Almere-FLEVOZIEKENHUIS
NL-Sittard-Geleen-ZUYDERLAND
NL-Alkmaar-NWZ
NL-Goes-ADRZ
NL-Dirksland-VANWEELBETHESDA
NL-Beverwijk-RKZ
NL-Amsterdam-SLAZANDREAS
