HOVON HO65 MM
Main info
- Identifier:
- HO65 MM
- Sponsor:
- HOVON
- Working group party:
- Myeloma
- Age:
- 18-65
- Stage:
- 1st Line
- Included patients:
-
833(of 833)
- Active sites:
-
82(of 76)
- Title:
A randomized phase III study on the effect of Bortezomib combined with Adriamycin, Dexamethasone (AD) for induction treatment, followed by High Dose Melphalan and Bortezomib alone during maintenance in patients with multiple myeloma
Timeline
News
This study has been closed for further inclusion on 16may08.
Flow
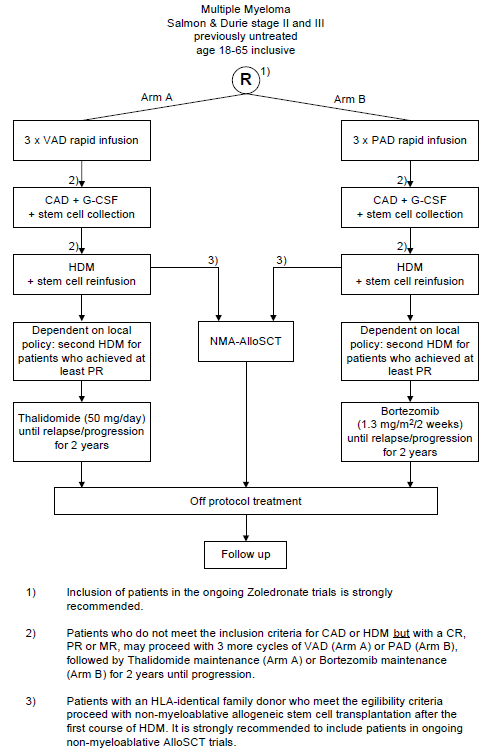
Details
- Phase:
- Prospective Phase III study
- Monitoring Type:
- Study Specific
- Objectives:
- To assess the efficacy of Bortezomib combined with intensive chemotherapy and in maintenance therapy in comparison with intensive therapy with Vincristine followed by thalidomide maintenance in patients with previously untreated multiple myeloma, as measured by the progression free survival as defined in chapter 14.
- To evaluate the overall response rate and CR + VGPR (complete and very good partial response) both after induction therapy and after autologous transplant.
- To evaluate overall survival.
- To assess the safety and toxicity of Bortezomib combined with intensive chemotherapy and in maintenance therapy.
- To assess the prognostic value of risk factors at diagnosis, including β2-microglobulin, karyotypic abnormalities of chromosomes 9, 11 and 13 as analyzed in bone marrow plasma cells by karyotyping and FISH, with respect to progression free survival.
- To analyze the prognostic value of myeloma gene expression profiles on the overall response on induction of all patients and of patients treated with Bortezomib separately.
Eligibility
- Inclusion Criteria:
- Patients with a confirmed diagnosis of multiple myeloma stage II or III according to the Salmon & Durie criteria (see appendix A);
- Age 18-65 years inclusive;
- WHO performance status 0-3 (WHO=3 is allowed only when caused by MM and not by co-morbid conditions) (see appendix D);
- Negative pregnancy test at inclusion if applicable;
- Written informed consent.
- Exclusion Criteria:
- Known intolerance of Thalidomide or Boron;
- Systemic AL amyloidosis;
- Non-secretory MM
- Previous chemotherapy or radiotherapy except 2 cycles of Melphalan/Prednisone or local radiotherapy in case of local myeloma progression;
- Severe cardiac dysfunction (NYHA classification II-IV, see appendix E);
- Significant hepatic dysfunction (serum bilirubin ≥ 30 μmol/l or transaminases ≥ 2.5 times normal level), unless related to myeloma;
- Patients known to be HIV-positive;
- Patients with active, uncontrolled infections;
- Patients with neuropathy, CTC grade 2 or higher
- Patients with a history of active malignancy during the past 5 years with the exception of basal carcinoma of the skin or stage 0 cervical carcinoma;
- Patients who are not willing or capable to use adequate contraception during the therapy (all men, all pre-menopausal women);
- Patients ≤ 65 years with an HLA-identical sibling who will undergo non-myeloablative AlloSCT;
- Lactating women.
Registration Details
The patient should be registered immediately after diagnosis, and before the start of chemotherapy. Patients need to be registered at the HOVON Data Center of the Erasmus MC Rotterdam location Daniel by phone call: +31.10.4391568 or fax +31.10.4391028 Monday through Friday, from 09:00 to 17:00 or via the Internet via TOP (Trial Online Process; http://www.hdc.hovon.nl/top). A logon to TOP can be requested at the HOVON Data Center for participants.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Patients initials or code
- Patients hospital record number (not obligatory)
- Sex
- Date of birth
- Serum β2-microglobulin value
- Serum albumin value
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
