HOVON HO69
Archived
Main info
- Identifier:
- HOVON 69 T-NHL
- Sponsor:
- HOVON
- Included patients:
-
20
- Active sites:
-
19(of 19)
- Title:
A phase II study of anti-CD52 monoclonal antibody (Alemtuzumab, MabCampath) with 2-weekly CHOP chemotherapy (Camp-CHOP 14) in patients with mature T-cell non-Hodgkin's lymphoma.
Timeline
Scheduled
Actual
2005
16 Nov
Activated
2007
01 Nov
CloseoutInProgressLastPtOutActualStart
2015
13 Oct
Archived
2030
13 Oct
Destruction
Flow
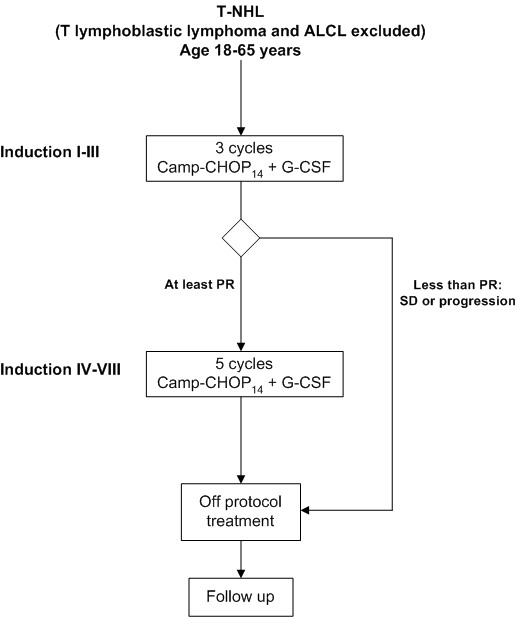
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Patients with a confirmed histologic diagnosis of T-NHL according to the WHO classification:
a. Extranodal NK/T cell lymphoma, nasal type;
b. Enteropathy-type T-cell lymphoma (EATL), if measurable disease;
c. Subcutaneous panniculitis-like T-NHL;
d. Angioimmunoblastic T-cell lymphoma;
e. Peripheral T-cell lymphoma, unspecified (T-NHL NOS);- Age 18-65 years inclusive;
- Ann Arbor stage II or more;
- WHO performance status 0, 1 or 2;
- Measurable disease;
- Written informed consent
- Exclusion Criteria:
- Patients with NK/T-NHL of the following type:
a. Precursor T cell lymphoblastic lymphoma/leukemia;
b. All mature T cell leukemias (T-PLL, ATLL, NK cell leukemia, T-LGL);
c. Anaplastic large cell lymphoma;
d. Hepatosplenic T cell lymphoma;
e. Enteropathy-type T cell lymphoma without measurable disease;
f. Blastic NK cell lymphoma;- Intolerance of exogenous protein administration;
- Severe cardiac dysfunction (NYHA classification II-IV) or LVEF < 45 %;
- Significant renal dysfunction (serum creatinine >= 150 mmol/l), unless related to NHL;
- Significant hepatic dysfunction (total bilirubin >= 30 mmol/l or transaminases >= 2.5 times normal level), unless related to NHL;
- Suspected or documented Central Nervous System involvement by NHL;
- Patients known to be HIV-positive;
- Patients with active, uncontrolled infections;
- Patients with uncontrolled asthma or allergy, requiring steroid treatment;
- Prior treatment with chemotherapy, radiotherapy or immunotherapy for this lymphoma, except local radiotherapy in case of (potential) organ dysfunction by localized lymphoma mass or infiltration;
- History of active cancer during the past 5 years, except basal carcinoma of the skin or stage 0 cervical carcinoma;
Participating Sites
Site
19 results
Order by
Accrual rate
Activation date
NL-Zwolle-ISALA
5
NL-Groningen-UMCG
4
NL-Rotterdam-ERASMUSMC
3
NL-Den Haag-HAGA
2
NL-Nieuwegein-ANTONIUS
1
NL-Maastricht-MUMC
1
NL-Roosendaal-BRAVIS
1
NL-Amsterdam-VUMC
1
NL-Amsterdam-AMC
1
NL-Leiden-LUMC
1
NL-Utrecht-DIAKONESSENUTRECHT
NL-Utrecht-UMCUTRECHT
NL-Rotterdam-EMCDANIEL
NL-Leeuwarden-MCL
NL-Enschede-MST
NL-Amsterdam-OLVG
NL-Amsterdam-AVL
NL-Amersfoort-MEANDERMC
NL-Amstelveen-AMSTELLAND
