HOVON HO74
Archived
Main info
- Identifier:
- HOVON 74 ALL
- Sponsor:
- HOVON
- Included patients:
-
2
- Active sites:
-
8(of 8)
- Title:
Alemtuzumab as remission induction for adult patients with acute lymphoblastic leukemia in relapse
A randomized phase II study.
Timeline
Scheduled
Actual
2006
11 May
Activated
2007
19 Oct
CloseoutInProgressLastPtOutActualStart
2017
19 Oct
Archived
2042
18 Oct
Destruction
Flow
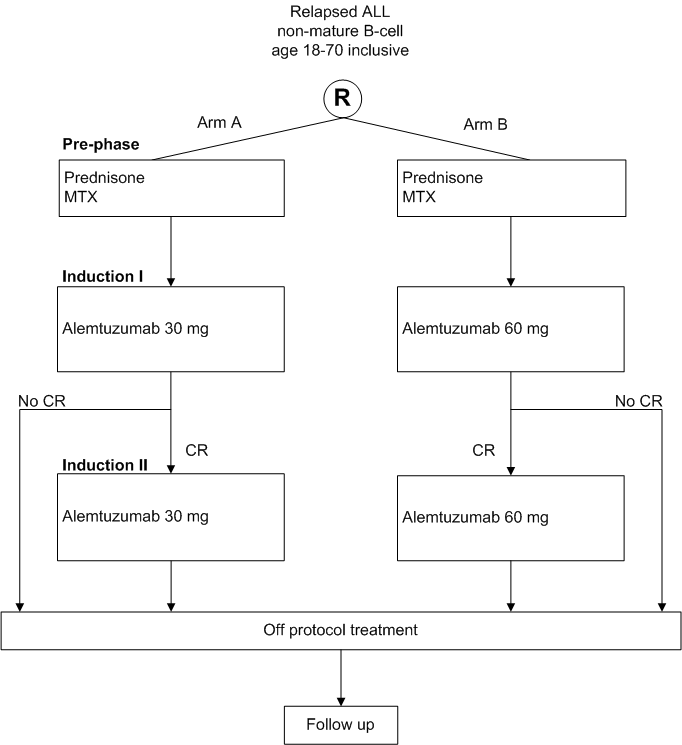
Details
- Phase:
- Prospective randomized Phase II study
- Monitoring Type:
- Objectives:
Eligibility
- Inclusion Criteria:
- Age 18 - 70 years inclusive
- First or second relapse of precursor B-ALL or T-ALL (including Philadelphia chromosome or BCR-ABL positive ALL)
- Duration of last complete remission at least 6 months
- WHO performance status 0, 1, or 2
- Negative pregnancy test at inclusion if applicable
- Written informed consent
- Exclusion Criteria:
- Mature B-cell ALL, i.e. Burkitt leukemia/lymphoma
- Acute undifferentiated leukemia (AUL)
- Treatment with alemtuzumab at any time prior to registration
- Intolerance of exogenous protein administration
- Central nervous system (CNS) leukemia (appendix A)
- Severe cardiovascular disease (arrhythmias requiring chronic treatment, congestive heart failure or symptomatic ischemic heart disease)
- Severe pulmonary dysfunction (CTCAE grade III-IV)
- Severe neurological or psychiatric disease
- Significant hepatic dysfunction (serum bilirubin or transaminases >= 3 times normal level)
- Significant renal dysfunction (serum creatinin >= 3 times normal level)
- Patients with active, uncontrolled infections
- Patients with uncontrolled asthma or allergy, requiring oral steroid treatment at the time of registration
- Patients known to be HIV-positive
- Patient is a lactating woman
- Any psychological, familial, sociological or geographical condition potentially hampering compliance with the study protocol and follow-up schedule
Participating Sites
Site
8 results
Order by
Accrual rate
Activation date
NL-Rotterdam-ERASMUSMC
1
NL-Leiden-LUMC
1
NL-Rotterdam-EMCDANIEL
NL-Amsterdam-AMC
NL-Amsterdam-VUMC
NL-Enschede-MST
NL-Maastricht-MUMC
NL-Nieuwegein-ANTONIUS
