HOVON HO75 NHL
Main info
- Identifier:
- HOVON 75 MCL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- 18-65
- Stage:
- 1st Line
- Echelon:
- Level C-SCT
- Included patients:
-
140(of 135)
- Active sites:
-
16(of 16)
- Title:
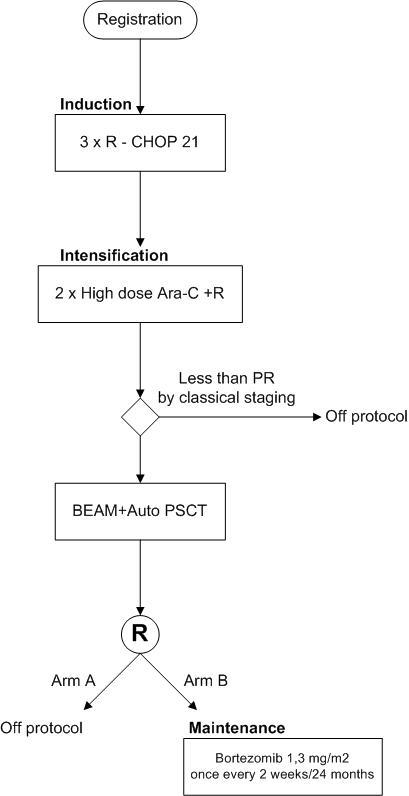
Bortezomib maintenance therapy in newly diagnosed patients with mantle cell lymphoma, responsive on rituximab combined with CHOP and high dose Ara-C and after BEAM with auto PSCT rescue.
Timeline
News
Sending in of material translational studies: As of today material for translational studies (MRD (only PCR-DNA) and proteomics) can be send to the department of Immunology of the Erasmus MC. Please use one form per sample to indicate which sample is send in (form can be found on this website).
Protocol: A new version of the protocol is available, version 4 (09SEP2010). Protocol has been amended for the following reasons:
increase of patients to include
deletion of namecode
update of risk section
update of safety section
CRFs: A new version (v7) of the CRFs (and instructions) is available. (Namecode has been deleted from header)
Drug order form: A new version of initial (v2) and re-supply (v3) form is available, this also accounts for the pharmacy info letter (v4).
Changes have been made to contact persons at Janssen Pharmaceuticals and the possibility of ordering per mail has been added.
Flow
Details
- Phase:
- Prospective randomized Phase II study
- Monitoring Type:
- Study Specific
- Objectives:
Primary objective
To study the efficacy and safety of bortezomib maintenance after induction with HD Ara-C and stem cell transplantation.Secondary objective
To study the value of FDG-PET, flow cytometry and IGVH–PCR for defining the quality of remission after induction with high dose chemotherapy and Rituximab, after auto PSCT and during maintenance treatment with bortezomib, in comparison to conventional definitions.
Eligibility
- Inclusion Criteria:
Patients with histologically and immunologically proven diagnosis of MCL (WHO classification)
- Ann Arbor stage II – IV
- CD20 positive
- Age 18 – 65 years (inclusive)
- WHO performance ≤ 2
- Measurable disease (also patients with isolated bone marrow disease are accepted) (appendix B)
- Written informed consent
Inclusion criteria for late randomization
- Completed BEAM treatment
- No progressive disease or relapse
- Neutrophils >0.5 x 10^9/l
- Platelets > 80 x 10^9/l
- Exclusion Criteria:
- Renal failure (creatinine clearance < 50 ml/min)
- Known hypersensitivity to murine antibodies, boron or mannitol
- Any other organ dysfunction or failure that may present a risk to the patient during any phase of protocol treatment.
- Presence of CNS involvement by NHL
- Known HIV and hepatitis B or C seropositivity
- Pregnancy or lactation
- Prior treatment with chemotherapy, radiotherapy or immunotherapy for this lymphoma, except local radiotherapy in case of (potential) organ dysfunction by localized lymphoma mass or infiltration
- Other active malignancy (less than 5 years in complete remission) except skin (nonmelanoma) or cervix carcinoma stage 1
- Active systemic infection requiring treatment
- Peripheral neuropathy or neuropathic pain Grade 2 or higher as defined by NCI CTCAE version 3
- Uncontrolled or severe cardiovascular disease, including MI within 6 months of enrolment, New York Heart Association (NYHA) Class III or IV heart failure, uncontrolled angina, clinically significant pericardial disease, or cardiac amyloidosis.
- Serious medical condition (such as severe hepatic impairment, pericardial disease, acute diffuse infiltrative pulmonary disease, systemic infections etc) or psychiatric illness likely to interfere with participation in this clinical study).
Exclusion criteria for late randomization
- Known hypersensitivity to murine antibodies, boron or mannitol
- Peripheral neuropathy or neuropathic pain Grade 2 or higher as defined by NCI CTCAE version 3
- Uncontrolled or severe cardiovascular disease, including MI within 6 months of enrolment, New York Heart Association (NYHA) Class III or IV heart failure, uncontrolled angina, clinically significant pericardial disease, or cardiac amyloidosis.
- Serious medical condition (such as severe hepatic impairment, pericardial disease, acute diffuse infiltrative pulmonary disease, systemic infections etc) or psychiatric illness likely to interfere with participation in this clinical study).
Registration Details
Eligible patients should be registered before start of treatment. Patients can be registered at the HOVON Data Center of the Erasmus MC – Daniel den Hoed via the Internet through TOP (Trial Online Process; https://www.hdc.hovon.nl/top/logon.asp). Alternatively patient can be registered by phone call: +31.10.7041560 or fax +31.10.7041028 Monday through Friday, from 09:00 to 17:00. A logon to TOP can be requested at the HOVON Data Center for participants.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Local patient code (optional)
- Sex
- Date of birth
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
