HOVON HO77 NHL
Main info
- Identifier:
- HO77 NHL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- >= 60
- Stage:
- 1st Line
- Included patients:
-
19(of 19)
- Active sites:
-
32(of 24)
- Title:
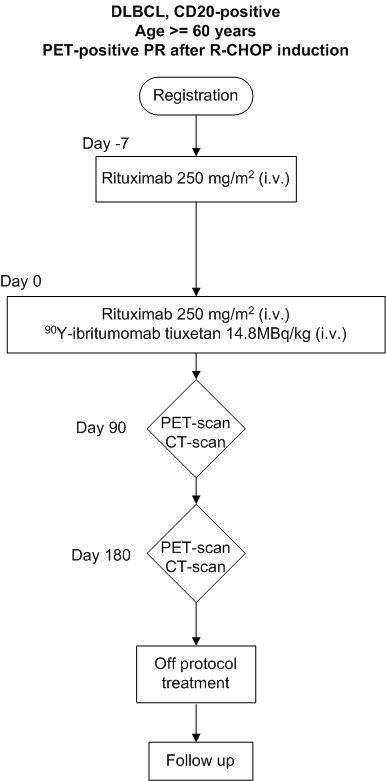
Efficacy and safety of a single dose of 14.8 MBq/kg (0.4 mCi/kg) 90Y-ibritumomab tiuxetan ('Zevalin') in elderly patients with diffuse large B-cell lymphoma and FDG-PET positive partial remission following first-line R-CHOP therapy. A Phase II clinical trial (HOVON 77)
Timeline
News
10 JUN 2010: Nieuwe versie CRFs staan op de website: versie 01 JUN 2010. Graag vanaf dit moment alleen deze nieuwe versie gebruiken voor alle patienten.
1 JUN 2010: De HOVON 77 studie is gesloten voor inclusie. De interim analyse (tevens eindanalyse) zal eind 2010 plaatsvinden.
4 FEB 2010: De HOVON 77 studie is nog steeds open !
Tijdens de HOVON meeting najaar 2009 is gemeld dat de HOVON 77 NHL studie eind december gesloten zou worden. Besloten is echter de studie open te houden tot de 20e patient is geincludeerd. Momenteel staat de teller op 18. Alle deelnemers zullen worden geinformeerd, wanneer de sluitingsdatum bekend is.
Flow
Details
- Phase:
- Prospective Phase II study
- Monitoring Type:
- Not any more
- Objectives:
Primary objective
The primary objective of this study is to evaluate the conversion rate from PET-positive to PETnegative residual masses after 90Y-ibritumomab tiuxetan treatment in patients with PET-positive partial remission following first-line R-CHOP chemotherapy.Secondary objectives
To evaluate the progression-free survival, the overall survival (OS) and the toxicity of DLBCLpatients with PET-positive partial remission treated with 90Y-ibritumomab tiuxetan.
Eligibility
- Inclusion Criteria:
- Age ≥ 60 years old
- WHO performance status of 0-2 (see Appendix E)
- Life expectancy of at least 3 months
- Histologically confirmed CD20 positive Diffuse large B-cell lymphoma (DLBCL), according to the WHO classification (see Appendix B)
- First-line induction treatment with R-CHOP or R-CHOP-like chemotherapy (only CHOP in combination with rituximab; CHOP14 and CHOP21 are both allowed)
- Partial response on CT-scans after first-line treatment, with measurable disease
- PET-positive residual mass
- Patient is not eligible for high dose chemotherapy followed by autologous stem cell transplantation
- Less than 25% bone marrow involvement at the end of first-line treatment during PR analysis (measurement in a representative bone marrow biopsy)
- Absolute neutrophil count (ANC) ≥ 1.5 x 10^9/l
- Hemoglobin (Hb) ≥ 6 mmol/l
- Platelets ≥ 150 x 10^9/l
- Written informed consent obtained according to local guidelines
- Exclusion Criteria:
- Hypoplastic bone marrow at biopsy
- Prolonged pancytopenia during induction chemotherapy and delayed courses during RCHOP induction (more than two weeks delay due to insufficient bone marrow reserve)
- Known hypersensitivity to murine antibodies or proteins
- Significant splenomegaly
- Patients with abnormal liver function (total bilirubin > 2.0 x ULN)
- Patients with abnormal renal function (serum creatinine > 2.0 x ULN)
- Presence of CNS involvement by NHL
- Presence of any other active neoplasms or history of prior malignancy, except nonmelanoma skin tumours or stage 0 (in situ) cervical carcinoma during the past 5 years
- More than one prior R-CHOP or R-CHOP-like chemotherapy regimen for DLBCL
- Patients who have received prior external beam radiotherapy to > 25% of active bone marrow (involved field or regional)
- Patients who have received G-CSF or GM-CSF therapy within two weeks prior to study enrollment
- Concurrent severe and/or uncontrolled medical condition (e.g. uncontrolled diabetes, congestive heart failure, myocardial infarction within 6 months of study, unstable and uncontrolled hypertension, chronic renal disease, or active uncontrolled infection) which could compromise participation in the study
- Patients who have received biologic therapy, immunotherapy, R-CHOP(-like) chemotherapy, surgery, or an investigational drugs less than 4 weeks prior to first day of study treatment or who have not recovered from the toxic effects of such therapy
- Patients who have received systemic corticosteroids at doses higher than 20 mg/day prednisolone or equivalent less than 2 weeks prior to 90Y-ibritumomab tiuxetan administration
- Known diagnosis of HIV infection
- Patients unwilling or unable to comply with the protocol
Registration Details
Eligible patients should be registered before start of treatment. Patients can be registered at the HOVON Data Center of the Erasmus MC - Daniel den Hoed by phone call: +31.10.4391568 or fax +31.10.4391028 Monday through Friday, from 09:00 to 17:00, or via the Internet through TOP (Trial Online Process; https://www.hdc.hovon.nl/top). A logon to TOP can be requested at the HOVON Data Center for participants.
The following information will be requested at registration:
- Protocol number
- Institution name
- Name of caller/responsible investigator
- Patients initials or code
- Patients hospital record number (optional)
- Sex
- Date of birth
- Eligibility criteria
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
