HOVON HO84 NHL
Main info
- Identifier:
- HO84 NHL
- Sponsor:
- HOVON
- Working group party:
- Lymphoma
- Age:
- 18-80
- Stage:
- 1st Line
- Echelon:
- Level D
- Included patients:
-
600(of 600)
- Active sites:
-
68(of 87)
- Title:
Randomized phase III study on the effect of early intensification of rituximab in combination with 2-weekly CHOP chemotherapy followed by rituximab maintenance in patients with diffuse large B-cell lymphoma
Timeline
News
Sept-2022:
The study has been archived.
Updated documents:
- 5 mar 2013: Nieuwe versie WMO-verklaring; Einddatum verzekering gewijzigd van dec2012 naar dec2014.
- 14 jan 2013: Nieuwe versie Rituximab Bestelfax.
April 10, 2012:
- The study is closed for inclusion of new patients, because the target (600 patients) has been reached. The randomization for maintenance treatment will not be closed before the last patient has reached this timepoint.
Updated documents:
- 12oct2011: a new version of the FAQ sheet is added.
- 6sep2011: Nieuwe versie CRFs (versie 5, 1SEP2011). In deze versie zijn 3 vragen uit het Informed Consent en 1 vraag over het nieuwe PK Informed Consent toegevoegd. Vanaf heden graag alleen deze versie gebruiken voor ALLE patienten. (Het SAE form is niet gewijzigd.)
6 sep 2011:
- Addendum Pharmacokinetic Evaluation Rituximab geaktiveerd (Amendement 5, alleen NL). Deze Rituximab PK Side Study in 40 HOVON84 patienten, zal slechts in 9 NL centra gaan lopen (Erasmus MC, VUMC, Amphia, Jeroen Bosch, Haga, Alb. Schweizer, UMCG, MCL Antonius). De overige NL/B/DK centra hoeven dus geen aktie te ondernemen. Alle PK specifieke documenten (PK Addendum, PK ICF template, METC goedkeuring amendement, Site Documents Checklist AM5:PK addendum) zijn vanaf vandaag te downloaden vanaf de website.
Updated documents:
- 7jul2011: FAQ voor datamanagers is toegevoegd.
- 22 jun 2010: Nieuwe versie SAE form (versie 03, 22JUN2010) en nieuwe versie SAE report instructions (versie 04, 27APR2010)
- 25 mar 2010: Nieuwe versie Flowchart Clinical Evaluations (versie AM1)
- 21 jan 2010: Nieuwe versie Rituximab Bestelfax.
- 13 okt 09: nieuwe versie CRFs (versie 4, 12okt2009). In deze versie zijn enkele fouten in het 'Registratie&Randomization Form (1)' gecorrigeerd.
Amendement 1:
- Amendement 1 is op 16 september 2009 goedgekeurd door de nederlandse centrale METC (Erasmus MC). De belangrijkste wijzigingen in dit amendement zijn:
- Het terugbrengen van het aantal twee-wekelijkse rituximab-CHOP kuren van 8 naar 6 voor oudere patienten (66-80 jaar) (Opm.: al aktief sinds juli 2009)
- Het includeren van jongere patienten (18-65 jaar) met een aa-IPI van 1-3
- Kleine wijziging in de centrale PET review procedure
- De evaluatie van de respons blijft op dezelfde momenten plaatsvinden, dus na 4 kuren en na de laatste kuur.
- Dit amendement is in Nederland vanaf donderdag 1 oktober 2009 aktief. Momenteel is het amendment ook in Belgie en Denemarken aktief. Dat betekent dat alle centra óók jongere patienten(18-65 jaar) mogen includeren. Het terugbrengen van het aantal R-CHOP kuren was al eerder geadviseerd (mailing juli 2009)
Flow
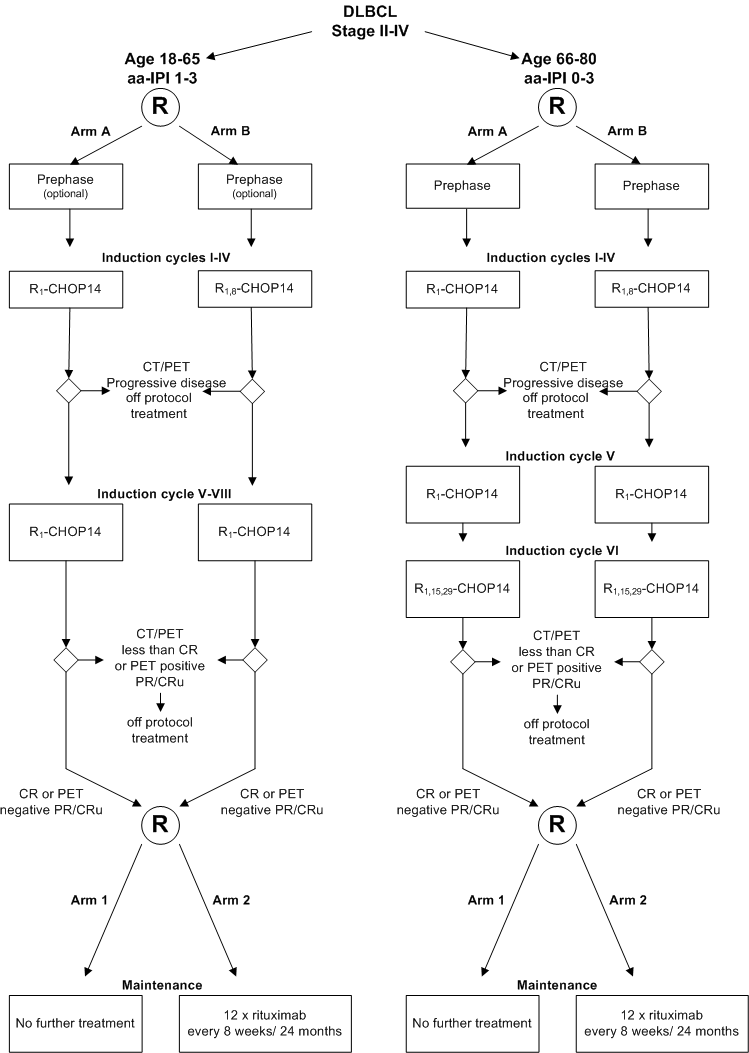
Details
- Phase:
- Prospective randomized Phase III study
- Monitoring Type:
- Not any more
- Objectives:
To assess in a prospective, multicenter, randomized phase III study in patients with DLBCL:
- The effect of early intensification of rituximab combined with 2-weekly CHOP +G-CSF (CHOP14) in comparison to no intensification of rituximab on the response rate (complete remission and FDG-PET negative partial remission/unconfirmed complete remission) and time to reach response
- The efficacy of maintenance treatment with rituximab in comparison to no further treatment on failure free survival
Eligibility
- Inclusion Criteria:
- Patients with a confirmed histologic diagnosis of diffuse large B-cell lymphoma (DLBCL) based upon a representative histology specimen according to the WHO classification (see protocol appendix A)
- DLBCL must be CD20 positive
- Ann Arbor stages II-IV (see protocol appendix C)
- Age 18-65 (inclusive) years and aa-IPI 1-3 (see protocol appendix G) OR Age 66-80 (inclusive) years and aa-IPI 0-3 (see protocol appendix G)
- WHO performance status 0 – 2 (see protocol appendix E)
- Written informed consent
- Exclusion Criteria:
- Age 18-65 (inclusive) years and aa-IPI 0 (no risk factors, see protocol appendix G)
- Intolerance of exogenous protein administration
- Severe cardiac dysfunction (NYHA classification III-IV, see protocol appendix F) or LVEF < 45%. Congestive heart failure or symptomatic coronary artery disease or cardiac arrhythmias not well controlled with medication. Myocardial infarction during the last 6 months
- Severe pulmonary dysfunction (vital capacity or diffusion capacity < 50% of predicted value) unless clearly related to NHL involvement
- Patients with uncontrolled asthma or allergy, requiring systemic steroid treatment
- Significant hepatic dysfunction (total bilirubin ≥ 30µmol/l or transaminases ≥ 2.5 x upper normal limit), unless related to NHL
- Significant renal dysfunction (serum creatinine ≥ 150 µmol/l or clearance ≤ 60 ml/min), unless related to NHL
- Clinical signs of severe cerebral dysfunction
- Suspected or documented Central Nervous System involvement by NHL
- Patients with a history of uncontrolled seizures, central nervous system disorders or psychiatric disability judged by the investigator to be clinically significant and adversely affecting compliance to study drugs
- Testicular DLBCL
- Primary mediastinal B cell lymphoma
- Transformed indolent lymphoma
- (EBV) post-transplant lymphoproliferative disorder
- Secondary lymphoma after previous chemotherapy or radiotherapy
- Major surgery, other than diagnostic surgery, within the last 4 weeks
- Patients with active uncontrolled infections
- Patients known to be HIV-positive
- Active chronic hepatitis B or C infection
- Serious underlying medical conditions, which could impair the ability of the patient to participate in the trial (e.g. ongoing infection, uncontrolled diabetes mellitus, gastric ulcers, active autoimmune disease)
- Life expectancy < 6 months
- Prior treatment with chemotherapy, radiotherapy or immunotherapy for this lymphoma, except a short course of prednisone (< 1 week) and/or cyclophosphamide (< 1 week and not in excess of 900 mg/m2 cumulative) or local radiotherapy in order to control life threatening tumor related symptoms
- History of active cancer during the past 5 years, except basal carcinoma of the skin or stage 0 cervical carcinoma
Registration Details
The patient should be registered immediately after satisfactory completion of screening tests and obtaining informed consent, and before the start of chemotherapy.
Patients need to be registered at the HOVON Data Center of the Erasmus MC - Daniel den Hoed
- by phone call: +31.10.7041560 or fax +31.10.7041028 Monday through Friday, from 09:00 to 17:00, or
- via the Internet through TOP (Trial Online Process; https://www.hdc.hovon.nl/top). A logon to TOP can be requested at the HOVON Data Center for participants.
Follow up 1x per jaar, tot 10 jaar na randomisatie.
Participating Sites
Ziekenhuizen die deelnemen aan het onderzoek staan benoemd op de HOVON website bij het onderzoek. Het kan zijn dat uw ziekenhuis niet genoemd wordt, maar wel aan het onderzoek deelneemt. Informeer hiernaar bij uw arts.
